When patients with haemophilia complicated by inhibitors undergo surgical procedures, they require careful management of their condition to achieve adequate haemostasis, both peri- and postoperatively. This is critical for patient recovery and successful surgical outcomes. Recombinant Factor VIIa (rFVIIa) is a bypassing agent used as surgical cover in inhibitor patients, and is administered in bolus infusion at regular intervals. For major surgical procedures with a high bleeding risk, it is commonly administered every two hours for the first 48 hours after surgery, then three-hourly for the next 48 hours, and thereafter four-hourly for another 72 hours [1].
This regimen is time consuming and demanding in terms of nursing staff resources. In the United Kingdom, recommended guidelines for the administration of each bolus dose of rFVIIa requires the presence of two registered nurses to prepare, check and administer the dose [2]. Assuming the process takes 10-15 minutes of two nurses’ time per dose, this represents approximately six nursing hours per 24 hours.
In addition, there are safety implications for such a frequent dosing regimen. On busy hospital wards, it is not uncommon for doses to be delayed or even missed through lack of time or shortage of suitable nursing staff. This can potentially lead to failure of haemostasis, excessive bleeding and, eventually, suboptimal surgical outcomes. Clinical decision-making, when it comes to the selection of a haemostatic agent, can be affected by these issues.
This led us to investigate alternative delivery methods for rFVIIa as surgical haemostatic cover. Continuous infusion has been investigated previously, but results have been only partially effective, and it has been hypothesised that it is the thrombin burst achieved with a bolus dose of rFVIIa that generates a stable haemostatic plug to inhibit further blood loss [3,4]. To generate a stable haemostatic plug, 90mcg/kg per rFVIIa bolus is required [5,6]. Evidence suggests the clinical activity and biochemical stability of rFVIIa is maintained after reconstitution over a 24-hour period up to 25°C, with no clinical complications [7,8,9]. The biochemical and microbiological stability of reconstituted rFVIIa has also been studied by Christensen et al. in the context of continuous infusion, with no detectable microbiological growth (aerobic or anaerobic) found during 24-hour storage in a syringe at room temperature [7].
The use of an infusion pump to deliver bolus doses of bypassing agent was identified as a potential new method for investigation. We evaluated the safety and efficacy of using the B-Braun Perfusor® Space syringe driver to administer frequent bolus infusions of rFVIIa to two patients with severe haemophilia A and inhibitors, who underwent three surgical procedures between them at our institution. The choice of the B-Braun Perfusor® Space was driven by its established reputation for safety within the intensive care unit (ICU) environment, as well as for its capacity to deliver bolus infusions. We used the Becton-Dickinson Plastipak 60-mL syringe, which is similar to that used by Christensen et al. No report of leaching was reported by the authors of that study [10].
Methods
Two patients (Patient 1 and Patient 2) with severe haemophilia A and high responding inhibitors were scheduled to undergo one major and two minor surgical procedures (see Table 1).
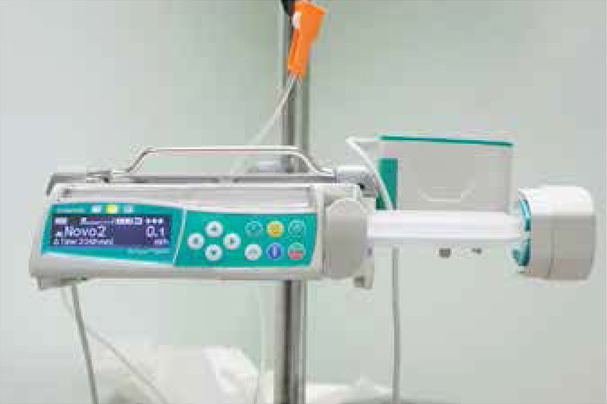
Clearance was obtained from the Trust medical electronics team and the senior nursing management to use the B-Braun Perfusor® Space syringe pump to deliver regular bolus doses of rFVIIa to provide haemostasis for the above procedures (see Figure 1). Haemophilia nurse specialists (HNSs) worked closely with the pump manufacturer to develop a programme, allowing the pump to deliver intermittent bolus infusions of rFVIIa with a choice of 90mcg/kg or 120mcg/kg, at two-, three- or four-hourly frequencies. The pump required a background continuous infusion of rFVIIa at all times, to ensure there was no blockage in the tubing and the tip of the cannula. The background infusion was set at 0.1ml/ hour for Patient 1, but reduced to 0.01ml/hour for Patient2. Haemostasis management was planned using a well-recognised consensus protocol as a basis [1].
Table 1
Patient characteristics and procedures
| PATIENT | AGE | FVIII | INHIBITOR TITRE | PROCEDURE(S) |
|---|---|---|---|---|
| 1 | 41 years | <1 IU/dl | 288 BU/ml | Total knee replacement |
| 2 | 52 years | <1 IU/dl | 8.3 BU/ml |
(a) Hickman line insertion (b) Formation of arteriovenous (AV) fistula |
There were two options for programming the pump. For Patient 1, the dosage was calculated according to his weight and this dose was programmed into the pump for administration. With Patient 2, his weight and required dose in mcg/kg was entered into the pump, which then automatically calculated the correct bolus dose of rFVIIa. The pump was programmed with three options for bolus delivery at two-, three- and four-hourly intervals to allow clinicians options to change the frequency of administration.
The infusions were made up by two HNSs in the haemophilia centre and taken to the patients’ bedside by the same nurses, ensuring compliance with legal and safety procedures. Once the process was completed, the nurses were able to leave as normal at the end of their shift, with one HNS remaining on call at all times in case of pump failure or other emergency. Ward nurses were responsible for monitoring and recording that the prescribed dosages were administered every two, three or four hours as specified, and a standard dose of 90 mcg/ kg was left with the ward staff to be given in the event of pump failure, to allow time for the HNS team to attend. The pump is fitted with an alarm system in the event of a malfunction; it can also be locked to prevent any inadvertent change to the dose. The syringe in the pump was changed every 24 hours by the HNS responsible for the programming of the dose and bolus frequency.
The syringe volume for each case was calculated around the HNSs’ shifts. For example, for Patient 1, on the day of surgery the volume was calculated to last from end of surgery (6pm) until mid-morning the following day. This allowed for clinical assessment and potential dose adjustment before replacing the syringe. Hospital policy allows for a maximum of 24 hours per infusion. The vials were reconstituted with 1ml/mg rFVIIa as per the SPC [10]. A mix of vial sizes were used to achieve the total volume desired. Total volume of rFVIIa given per bolus was less than the volume used if doses were rounded up.
Results
Patient 1 was a 41-year-old adult male admitted for total knee replacement (TKR) surgery. He had a PICC (peripherally inserted central catheter) line inserted beforehand to facilitate intravenous access under cover of rFVIIa. On the day of surgery, pre-operatively in the anaesthesia room, he was given the first bolus dose of 120mcg/kg of rFVIIa, followed by a second 90mcg/kg dose two hours later. Once in postoperative recovery, the patient received regular bolus infusions via the pump: doses were 90mcg/kg every two hours for 60 hours, followed by 90mcg/kg three-hourly for the next 69 hours, and finally four-hourly for 72 hours (see Table 2). The patient received IV fluids via the PICC line in the immediate postoperative period. These were discontinued once the patient was well hydrated and able to drink, after which a continuous infusion of normal saline at 20ml/ hour was run alongside the rFVIIa to maintain patency and prevent thrombosis at the catheter tip.
Table 2
rFVIIa bolus doses delivered via infusion pump
Patient 1’s procedure and subsequent recovery went smoothly, with no issues arising either with the infusion pump or with venous access. rFVIIa dosages were all delivered by the pump as programmed, with no need for intervention from the haemophilia team. The patient’s recovery was unremarkable and total use of rFVIIa did not exceed planned levels. The infusion pump was stopped after the fourth day to allow for patient mobilisation, and doses of rFVIIa were administered broadly in line with the protocol by the nursing team until discharge at day 14 (NB: this is a considerably longer hospital stay than would be required for a non-inhibitor patient, but standard practice for a case such as this). He continued to receive doses of rFVIIa as prophylaxis prior to each session of physiotherapy, in line with the centre’s standard protocol for joint replacement surgery in people with inhibitors. The patient continues to do well and his mobility and confidence have significantly improved. He has been able to return to full-time work and reports that he is pain-free.
Patient 2 was a 52-year-old adult male who required surgery for the formation of an arteriovenous (AV) fistula. The day before the procedure, he attended the centre to have a Hickman line inserted, and haemostasis was managed with the aid of the infusion pump. The patient received two-hourly bolus doses of rFVIIa over 24 hours, and the whole process went smoothly.
The following day, Patient 2 returned for his AV fistula surgery. The pre- and peri-operative doses of rFVIIa were delivered as with Patient 1: he received an initial bolus dose of 120mcg/kg in the anaesthesia room, and a second 90mcg/kg dose after two hours. Postoperatively, a similar process was followed to that with Patient 1: the pump was programmed to deliver regular bolus infusions of 90mcg/kg every two hours for 48 hours, then three-hourly for 72 hours, four-hourly for 24 hours, and finally six-hourly for 48 hours (see Table 2).
Once again, the patient’s recovery was smooth and unremarkable, and as with Patient 1, the total rFVIIa used did not exceed the planned amount. The background continuous infusion of rFVIIa was reduced to just 0.01ml/ hour with Patient 2, having set the pump at the higher level of 0.1ml/hour for Patient 1. In addition, the infusion line for this patient was replaced from a standard adult one to a paediatric line, which helped control the rate of delivery and further minimised waste.
Discussion
Our experience with the B-Braun Perfusor® Space syringe driver to deliver frequent intermittent bolus infusions of rFVIIa has proved a positive one, with excellent results and no adverse events. The pump allowed specialist staff to be in control of the administration of rFVIIa at all times, even when not on duty. The weight-based dose calculated by the pump achieved greater accuracy than traditional bolus dose preparation, where whole vials are traditionally used regardless of overall volume required. The pump also guaranteed the exact timing of each bolus dose, eliminating delays due to staff shortages and preventing missed doses.
As well as enhancing accuracy of dosing, the use of the pump allowed for considerable savings in nursing time. Based on a calculation that each bolus dose administration would require 10-15 minutes of two nurses’ time, i.e. six nursing hours per 24 hours, the time required by using the pump can be significantly reduced: with two nurses spending about 30 minutes drawing up 12 doses for 24 hours, this amounts to a total nursing time of just one hour per 24 hours.
The potential for wastage of rFVIIa dose was an issue with the first procedure, involving Patient 1. The background continuous infusion was set at a rate that, in hindsight, was unnecessarily high. However, this was rectified with Patient 2 by significantly reducing the rate and switching to a paediatric administration line.
The volume of rFVIIa wastage from the continuous infusion, in the case of Patient 1, was calculated at 0.1ml/hour per 24 hours with five syringe changes over five days, representing a total loss of 12ml (12mg). This procedure was carried out using a standard giving set with a volume of 4ml. Line changes are determined by Trust intravenous administration policies, which required that the line be changed every 72 hours. Line changes accounted for a further loss of 8ml (8mg). With Patient 2, rFVIIa wastage was reduced by adjusting the background infusion rate to 0.01ml/hour. This represents a loss of 0.24ml (0.24mg) per 24 hours, also with a total of five syringe changes in a five-day period. Total loss in this case was therefore just 1.2ml (1.2mg). The giving set for Patient 2 was changed to a fine bore set with a total volume of only 2ml, which was changed twice leading to a further loss of 4ml (4mg).
Overall, rFVIIa wastage was 20ml (20mg) for Patient 1, which would be considered excessive. However, following a change in practice, wastage for Patient 2 was just 5.2ml (5.2mg). There is no ‘standard’ level of wastage for bolus administration that could be used as a reference, but we believe the level achieved with Patient 2 was well within an acceptable range.
Additionally, as the pump is able to deliver an accurate bolus dose according to the patient’s weight, on each occasion there was a saving against traditional bolus dosing, where the dose would normally have been ‘rounded up’ to a whole vial of rFVIIa. In Patient 1, we estimate a further 4.7mg would have been administered using the conventional regimen. In Patient 2, there was a more marked difference. In our clinical practice, with traditional bolus dosing, this patient would have received 7mg per dose, whereas the accuracy afforded by the pump meant that each dose was exactly 90mcg/kg (6.3mg), resulting in an overall saving of 50.4mg of rFVIIa.
Haemostasis was unaffected by use of the pump, and no concerns arose over the stability or safety of rFVIIa when delivered in this way. Although bolus injection is the currently recommended regimen for rFVIIa use in surgical procedures, there is an unmet need for a more convenient and less time-consuming dosing regimen and means of administration.
Medical and nursing staff and patients expressed confidence in the performance of the perfusor pump.
The HNSs, although initially concerned about venous access, were reassured by the benefit of using a central line for dose delivery. Peripheral IV access may lead to some tissue damage – e.g. during some minor procedures – so use of a central line is advisable whenever possible. However, HNSs welcomed the fact that using the pump allowed them to remain in control of the haemostasis management of their patients, unlike with traditional bolus delivery when timely and accurate dosing is often in the hands of ward nurses.
The two patients, who had been admitted to hospital before and treated with traditional frequent bolus doses, reported feeling very confident with the use of the pump. They welcomed the fact that there was no need for them to be woken every two or three hours during the night for their next dose. One of the patients felt reassured by the timed delivery of his bolus doses, which meant he did not need to worry about the possibility of delayed doses due to ward staff being busy.
Conclusions
The use of a syringe pump to deliver frequent intermittent bolus doses of rFVIIa for patients with severe haemophilia A and inhibitors was found to be safe and effective in two patients undergoing a total of three surgical procedures. There was a significant saving in nursing time, allowing two haemophilia nurse specialists to draw up 24 hours’ worth of doses in just one session, as opposed to two nurses having to prepare, check and administer frequent (two-, three- or four-hourly) doses over 24 hours. We have demonstrated that, in the majority of cases, the small amount of wastage from using lines with an infusion pump is offset by the savings afforded by accurate dosing and – at least in the UK – by the reduction achieved in qualified nursing time. The pump also allows specialist staff to control rFVIIa administration at all times, and ensures the accuracy and correct timing of every dose, eliminating the potential for delayed or missed doses. While the number of patients studied so far on the pump is small, we believe this is an innovative and promising delivery mechanism for rFVIIa in an in-patient surgical setting, and it merits further investigation.