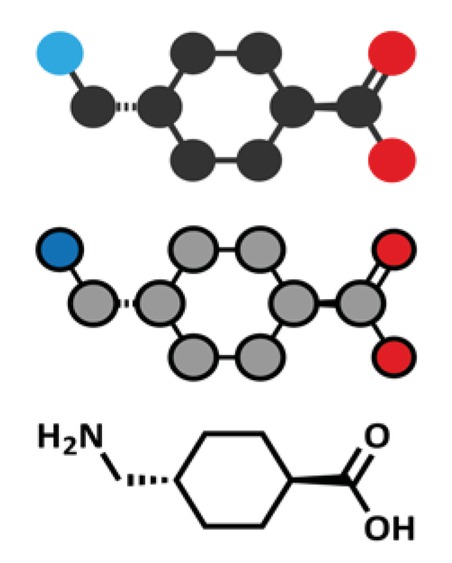
Tranexamic acid is the trans isomer of p-aminomethyl cyclohexane carboxylic acid (Figure 1; C8H15NO2; molecular weight 157 [1,2]). It is a white or almost crystalline powder, freely soluble in water and glacial acetic acid, but practically insoluble in acetone or ethanol. The original report on the synthesis of tranexamic acid was published by Okamoto and Okamoto in 1962 [50].
Pharmacokinetics
After oral administration of 10 or 100 mg/kg (the normal therapeutic dose is 15 – 25 mg/kg), peak serum concentrations of 2 mg/l and 40 mg/l respectively occur at approximately 3 – 4 hours [1]. Bioavailability is approximately 40%.
The distribution of tranexamic acid is described by a two-compartment pharmacokinetic model [1,3]. Plasma protein binding at therapeutic levels is about 3% and is due to binding to plasminogen; it does not bind to serum albumin. The volume of distribution is 9 – 12 litres [3,4]. Tranexamic acid diffuses rapidly into joint fluid and the synovial membrane, achieving concentrations similar to serum levels; the biological half-life in joint fluid is approximately 3 hours [1,4].
Tranexamic acid does not undergo metabolism and is excreted unchanged, predominantly in urine [4].
Tranexamic acid is excreted by glomerular filtration. Renal clearance in adults has been estimated at 80 ml/min/70kg3 (in patients undergoing cardiopulmonary bypass) and 110 - 116 m/min (not adjusted for body mass) [4]. In children with a mean age of 0.56, 5.5 and 36 months undergoing cardiopulmonary bypass, clearance was estimated at approximately 40, 84 and 170 ml/min [5].
The elimination half-life is about 3 hours in adults [4] and 1 – 2 hours in infants and children [5]. After a single intravenous dose of 10 mg/kg in adults, about 30% was recovered in the urine in the first hour, 55% up to 3 hours and 90% within 12 – 24 hours [1,4]. After oral administration of 10 – 15 mg/kg in adults, about 40% of the dose was recovered in the urine within 24 hours [1,4]. Plasma concentrations of tranexamic acid are increased in patients with renal insufficiency.
Mechanism of action and pharmacodynamic effects
Tranexamic acid inhibits fibrinolysis by competitively blocking the lysine binding sites of plasminogen, inhibiting binding between fibrin and plasminogen, and activation of plasminogen. It also competitively inhibits tissue plasminogen activator and inhibits plasmin-induced platelet activation [6,7]. In experimental models, tranexamic acid inhibits fibrinolysis over the concentration range 10 – 100 micromol/l and reduces bleeding time [8]. Adding tranexamic acid to recombinant FVIIa or FVIII increases clot stability to a much greater extent than the factors alone [9,10].
Formulations
There are many brands of tranexamic acid available globally. For example, it is marketed in the United States and Australia as Lysteda tablets, and as Cyklokapron and Transamin intravenous injection; in the UK as Cyklokapron (tablets and IV injection), Cyklo-F, Menstralite and Femstrual (all tablets); in Asia as Transcam (tablets); in Bangladesh as Traxyl (capsules, IV injection); in India as Pause (tablet); in South America as Espercil (capsule, IV injection); in Japan as Nicolda (tablets); in France and Romania as Exacyl (IV injection); and in Egypt as Kapron (tablets, IV injection). A capsule formulation is marketed in the Philippines as Hemostan and as Hexakapron in Israel [2]. Some brands are intended for prescription use only, others for over-the-counter or online sale. In Uganda, tranexamic acid is available as Transamin injection, Transamin 250 and 500mg tablets, Hemsamic 500mg tablets, Tranlok 500mg tablets and Tranlok injection [11]. There are also many generic formulations of oral and intravenous preparations; for example, MedIndia’s database lists 95 generic formulations manufactured by 65 companies [12].
Stability and storage
Information about correct storage for most formulations is not readily available. For the Cyklokapron brand, marketed by Pfizer in the UK, the Summary of Product Characteristics (SPC) for the injection states that its shelf life is 3 years; it does not recommend a temperature range for storage, but adds that it should not be frozen [13]. In the US, where Cyklokapron is marketed jointly by Pfizer and Pharmacia & Upjohn, the product labelling states that the injection should be stored at 25°C, but excursions are permitted within the range 15 – 30°C [14]. A US study has shown that tranexamic acid injection is stable for 12 weeks at temperatures of -20°C, 4°C, 22°C and 50°C, although the ampoules broke when the solution froze [15].
The ampoules are for single use. The chemicophysical stability of the solution is at least 24 hours at 25°C, but the solution contains no preservative and sterility cannot be assured after opening [13]. Cyklokapron injection can be mixed with most solutions for infusion (e.g. electrolyte, carbohydrate, amino acid and dextran solutions); heparin may be added to Cyklokapron injection [13]. If the injection is mixed with other products for intravenous administration, tranexamic acid is stable for up to 4 hours at room temperature [16]. Cyklokapron injection should not be mixed with blood or solutions containing a penicillin [13,16].
The UK SPC for Cyclokapron tablets states that the shelf life is 5 years; the tablets should be stored below 25°C [17]. In the US, Ferring Pharmaceuticals advise that Lysteda tablets should be stored at 15 – 30°C [16]. The same recommended temperature range is cited by MedIndia for the storing of both tablet and injection formulations [18], suggesting they are applicable globally. Differences in these recommended temperature ranges may reflect local custom and practice rather than evidence of stability.
No special disposal requirements have been found.
Although many online sites sell tranexamic acid, no reports of counterfeits of products intended for therapeutic use have been found (at least in the English language).
Tranexamic acid appears to be an ingredient in skin-whitening products and online postings suggest a degree of concern about fake formulations. However, it is unclear whether this is an important issue and online comments cannot be independently verified.
Indications and doses
The indications and doses licensed in the UK [13,17] have been used for the purpose of this review, on the basis that the data are reliable and the disposition of tranexamic acid is not affected by ethnicity.
Licensed indications in the UK
Cyklokapron tablets are licensed for short-term use for haemorrhage or risk of haemorrhage in increased fibrinolysis or fibrinogenolysis, and local fibrinolysis as it occurs in prostatectomy and bladder surgery, menorrhagia, epistaxis, conisation of the cervix, traumatic hyphaema and hereditary angioneurotic oedema. Of specific relevance to this paper, the tablets are also licensed for the ‘management of dental extraction in haemophiliacs’ [17].
Cyklokapron injection is not specifically licensed for an indication in people with haemophilia, but nor are they excluded. The licensed indications are: haemorrhage caused by general or local fibrinolysis (such as menorrhagia and metrorrhagia), gastrointestinal bleeding, haemorrhagic urinary disorders further to prostate surgery or surgical procedures affecting the urinary tract, surgery (specifically adenoidectomy, tonsillectomy, dental extractions; gynaecological or disorders of obstetric origin; thoracic, abdominal and other major surgical intervention such as cardiovascular surgery), and the management of haemorrhage due to the administration of a fibrinolytic agent [13].
Dosage
The recommended oral dose for the management of dental extraction in people with haemophilia is 2 – 3 500mg tablets every 8 hours (based on a dose of 25mg/kg/day).
The recommended intravenous dose as the standard treatment of local fibrinolysis is 0.5 – 1.0g by slow IV injection (at the rate of 1ml/minute) 2 – 3 times daily. For general fibrinolysis, the recommended dose is 1.0g by slow IV injection every 6 – 8 hours, which is equivalent to 15 mg/kg.
Contraindications and prescribing cautions
All contraindications and prescribing cautions listed in UK SPCs for Cyklokapron tablets and injections are shown in Tables 1 and 2; it should be noted that some are not relevant to people with haemophilia.
Table 1
Contraindications and precautions listed in UK SPCs for Cyklokapron tablets [17]
Table 2
Contraindications and precautions listed in UK SPCs for Cyklokapron injections [13]
Age
No dose adjustment is recommended for older people, except according to renal function.
Therapeutic experience is limited in children. The recommended oral dose is 25mg/kg per dose. The recommended intravenous dose is 20mg/kg/day.
Renal impairment
The use of tranexamic acid is contraindicated in patients with severe renal impairment. The oral dose should be reduced to 15mg/kg twice daily in patients with mild renal impairment (serum creatinine 120 – 249micromol/l) and to 15mg/kg once a day in moderate renal impairment (serum creatinine 250 – 500micromol/l). For patients with mild to moderate renal impairment, the intravenous dose is adjusted according to the serum creatinine level, as shown in Table 3.
Hepatic impairment
No dose adjustment is recommended for people with hepatic impairment. This is explicitly stated only for the injection in UK guidance, but should also apply to the oral formulation.
Pregnancy and breastfeeding
There is no evidence from animal studies that tranexamic acid is teratogenic. However, it crosses the placenta and is present in cord blood at concentrations equal to blood levels [16]. There is a lack of evidence showing that it is safe to take during the first trimester. There is no evidence that its use in women during the second and third trimesters is associated with adverse outcomes.
In the US, tranexamic acid is classed as Pregnancy (Category B), i.e. ‘Animal reproduction studies have failed to demonstrate a risk to the foetus and there are no adequate and well-controlled studies in pregnant women’ [19].
In the UK, Pfizer recommends that women of childbearing potential must use effective contraception during treatment with the injection, and that its use is not recommended during the first trimester. For the tablets, it recommends only that ‘usual caution… should be observed’.
There is no evidence that tranexamic acid affects fertility.
Tranexamic is excreted in breast milk at a concentration of 1% of the concurrent blood level after oral administration. An antifibrinolytic effect is unlikely. Pfizer in the UK recommends avoiding breastfeeding during treatment with the injection. In the US, Pfizer and Pharmacia & Upjohn advise caution [16]. A case-control study of outcomes in 21 women taking tranexamic acid while breastfeeding reported no acute adverse effects on the infant and no developmental effects [20].
Sources of evidence of efficacy
The medical and surgical risks associated with haemophilia are summarised in the 2012 update of the World Federation of Hemophilia (WHF) guideline [21]. A large number of clinical trials evaluating the efficacy of tranexamic acid have been published, but few prospective randomised comparative trials have involved people with haemophilia, in whom the balance of risk and benefit is fundamentally different from that in people with no bleeding disorder. This section summarises recent relevant evidence by indication, beginning with a precis of the WHF recommendations for the use of tranexamic acid.
WHF guideline [21]
The WHF recommendations for use of tranexamic acid are summarised in Appendix 1. Where WHF makes statements about clinical practice, it specifies the level of supporting evidence in which treatment benefits and harms are rated on a scale from 1 (best) to 5 (worst). Most of the evidence supporting the recommended indications for tranexamic acid is level 4 or 5. Full details are available at www.cebm.net/ocebm-levels-of-evidence
Dental or oral surgery (meta-analysis)
A Cochrane systematic review identified only one randomised controlled trial of tranexamic acid to prevent bleeding in people with haemophilia undergoing oral or dental surgery among 313 publications retrieved in a comprehensive literature search carried out in September and December 2015 [9,23]. The findings of this trial, which was reported in 1972 and was based on data collected in the 1960s, are summarised in Appendix 2. There were no eligible publications involving people with von Willebrand disease.
The appraisal of the trial concluded it was of moderate quality, largely due to its small size and incomplete documentation of randomisation and blinding. This trial suggested that, compared with placebo, 1.5 patients would need to be treated with tranexamic acid to prevent one case of postoperative bleeding.
Publications that do not meet the eligibility criteria for the Cochrane review include two small randomised controlled trials, [24,25], four retrospective studies [26, 27, 28, 29], one case-control study [30], and two case series [31,32]. These studies evaluated the effectiveness of a mouthwash to prevent bleeding from tooth descaling [24,25], compression with tranexamic acid [28], prophylaxis with tranexamic acid [26,27,30], perioperative use [31,32], or an unspecified regimen [29]. In several studies, tranexamic was not the only haemostatic agent used.
Surgery
A Cochrane systematic review (search date November 2014) identified no comparative randomised trials of sufficient quality for the use of tranexamic acid for bleeding prevention during surgery in people with haemophilia or other congenital bleeding disorders (other than that cited in the review of dental procedures above) [33].
Two papers were published after the literature search for the systematic review was carried out. One reviewed evidence for combining tranexamic acid and anti-inhibitor coagulant complex (AICC) in patients with inhibitors [34]. This combination is potentially synergistic and may increase clot stability, but it may also be associated with an increased risk of thrombotic events and disseminated intravascular coagulation. The authors found no reports of thrombotic events and concluded: ‘concomitant therapy with AICC and tranexamic acid can be considered when haemophilia patients with inhibitors undergo dental or surgical procedures to reduce blood loss and avoid life threatening bleeding. Furthermore, concomitant use of AICC and tranexamic acid should be considered for patients with bleeding who have failed monotherapy with either AICC or rFVIIa.’
However, evidence was based on low numbers of patients for some indications, and there is a need for further randomised controlled trials to provide stronger evidence.
An older, non-comparative study described treatment with the combination of tranexamic acid and APCC in a total of eight patients with congenital haemophilia with inhibitors or acquired haemophilia [35]. The haemostatic outcome was rated excellent or good in 10 of 11 treatments and poor in one. There were no episodes of arterial, venous thrombosis or disseminated intravascular coagulation, nor any laboratory signs of hypercoagulability.
The second recent paper analysed 56 surgical procedures in 40 patients with congenital or acquired haemophilia, who were treated primarily with recombinant activated factor VII [36]. Tranexamic acid was also administered in 64% of procedures, but the indications for its use are not reported and outcomes in this group of patients are not analysed separately.
Other publications do not substantially improve the evidence base. A structured review of cardiac surgery in people with bleeding disorders including haemophilia, who were treated with factor replacement therapy and antifibrinolytics including tranexamic acid, found a lack of good quality evidence and possible publication bias due to under-reporting of adverse outcomes [37]. A report on the use of tranexamic acid in orthopaedic procedures for people with rare bleeding disorders in general, and von Willebrand disease in particular, included several cases involving tranexamic acid [38.39]. A treatment protocol developed for four patients with severe FXI deficiency and inhibitors included prophylaxis with tranexamic acid and recombinant FVIIa [40]. The use of topical tranexamic acid to control perioperative bleeding was described in two women undergoing gynaecological surgery, one of whom had severe FIX deficiency (the other had thrombocytopenia) [41]. A retrospective review described the use of oral tranexamic acid with fibrin glue as part of a protocol to prevent bleeding complications associated with circumcision in boys with haemophilia [42]. In 28 people with mild to moderate inherited bleeding disorders undergoing endoscopy and receiving prophylaxis with tranexamic acid but no factor replacement therapy, there were three cases of bleeding [43].
Menorrhagia
No meta-analysis of studies of tranexamic acid in the management of menorrhagia in women with inherited bleeding disorders was found.
A recent survey of US haemophilia centres found that 62% of 1,321 women with von Willebrand disease attending
clinics between 2011 and 2014 had menorrhagia; the most common first-line treatments were combined oral contraceptives, tranexamic acid and desmopressin [44]. A retrospective review of the management of 42 girls with bleeding disorders who had menorrhagia reported that treatment with tranexamic acid, desmopressin, combined oral contraceptive pills, clotting factor concentrate and the levonorgestrel intrauterine system, alone or in combination, reduced menstrual blood flow and improved quality of life scores [45]. A review of the management of menorrhagia in women with inherited bleeding disorders places tranexamic acid among first-line therapies, along with combined oral contraceptives and the levonorgestrel intrauterine system; desmopressin is recommended when these options are inappropriate [46]. A randomised trial of tranexamic acid versus desmopressin included 116 women with menorrhagia and abnormal clotting times, of whom 14 had von Willebrand disease and 22 had ‘subnormal coagulation factor level’. Overall, tranexamic acid reduced blood flow more effectively and both treatments improved quality of life scores, but separate analysis by clotting disorder was not reported [47].
Adverse effects
The adverse effects of tranexamic acid are not well documented in people with haemophilia or other inherited bleeding disorders.
In 2012, the European Medicines Agency reviewed the efficacy and safety of tranexamic acid as part of a review of fibrinolytic drugs, and its findings are largely reflected in European and US labelling [48]. However, it concluded:
‘In view of the identified serious limitations of the efficacy data for treatment of haemophilia including the new data and the adverse reactions profile (some of which are serious) associated with the use of TXA [tranexamic acid], the CHMP Committee [Committee for Medicinal Products for Human Use] considers that the benefit risk balance of TXA in this indication is not positive under normal conditions when specific reference is made to haemophilia.’
By contrast, the WHF guideline, also published in 2012, believes the balance of risk and benefit is favourable for specific indications (see Appendix 1) [21].
The adverse effects listed by the current UK SPCs for Cyklokapron tablets and injection in the UK are shown in Table 4. This is broadly consistent with the US product labelling, which adds that convulsions are particularly associated with cardiovascular surgery and with inadvertent administration into the neuraxial system [14]. Estimates of the incidence of seizures associated with tranexamic acid in patients undergoing cardiac surgery range from 0 – 3% at standard doses and up to 7% at high doses [49]. Tranexamic acid may increase the excitability of neural networks, an effect that correlates with persistently high postoperative concentrations in cerebrospinal fluid. This raises the possibility of a risk of seizures in people with haemophilia.
Table 4
Adverse effects listed by the current UK SPCs for Cyklokapron tablets (17) and injection [17]
| Tablets [17] | Injection [13] | |
|---|---|---|
| Immune system disorders | Very rare: Hypersensitivity reactions, including anaphylaxis | Frequency not known: Hypersensitivity reactions, including anaphylaxis |
| Eye disorders | Rare: Colour vision disturbances, retinal/artery occlusion | Frequency not known: Visual disturbances, including impaired colour vision |
| Vascular disorders | Rare: Thromboembolic events Very rare: Arterial or venous thrombosis at any sites | Frequency not known: Malaise with hypotension, with or without loss of consciousness (generally following a too fast intravenous injection, exceptionally after oral administration). Arterial or venous thrombosis at any sites. |
| Gastro-intestinal disorders | Very rare: Digestive effects, such as nausea, vomiting and diarrhoea, may occur but disappear when the dosage is reduced. | Common: Diarrhoea, vomiting, nausea |
| Skin and subcutaneous tissue disorders | Rare: Allergic skin reactions | Uncommon: Dermatitis allergic |
| Nervous system disorders | - | Frequency not known: Convulsions, particularly in case of misuse |
Tranexamic acid may also be associated with impairment of mental and/or physical abilities [14]. Although European and US product labelling includes warnings about thromboembolic events [13,14,17], there is no information about how this risk applies to people with bleeding disorders.
Conclusion
In summary, tranexamic acid may be associated with a relatively narrow range of adverse effects, some of which are potentially serious. Their frequency in patients overall and in people with haemophilia is not clear. This uncertainty led the European Medicines Agency to conclude that the balance of risk and benefit is not positive in people with haemophilia, but this is not consistent with the recommendations of the WHF.