Intravenous factor VIII (FVIII) is the standard of care for the prevention of haemarthrosis and spontaneous bleeding episodes in persons with severe haemophilia A (HA). However, a third of persons with HA can develop neutralising antibodies to exogenous FVIII, termed inhibitors, reducing its activity and causing loss of haemostasis[1]. These inhibitory antibodies have been associated with increased morbidity and mortality and there has been a major unmet need for novel therapeutic agents[2]. Emicizumab is a bispecific, humanised, monoclonal antibody mimicking FVIII cofactor activity in mediating the generation of FXa by FIXa in persons with severe HA[3]. This subcutaneous non-factor agent has been approved as a prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and children (ages newborn and older) with HA with or without FVIII inhibitors[4], although few data are currently available on children.
The efficacy and safety of emicizumab was assessed in several phase 3 clinical trials[5,6,7,8]. Prophylactic treatment with emicizumab significantly reduced bleeding rates in adolescents and children with HA with or without FVIII inhibitors, and was generally well tolerated. In addition, subcutaneous administration of emicizumab provided beneficial effects on health-related quality of life (HRQoL) and lessened the burden of the disease in persons with HA as well as in their caregivers[9].
While data on the clinical outcomes of the management of persons with HA in real-world settings are limited, data from the DOSE study showed that in severe haemophilia with inhibitors, bleeding episodes interfered with the daily activities of persons with haemophilia and their caregivers. In addition, bleed days were associated with significantly worse HRQoL[10]. More data on the clinical outcomes of emicizumab, particularly in terms of reducing bleeding rates and improving HRQoL, would guide the appropriate positioning of this agent among haemophilia management approaches.
In this context, the purpose of this observational and retrospective study was to review the clinical outcomes of emicizumab prophylaxis in children and adults with severe haemophilia at two tertiary hospitals in the United Arab Emirates (UAE), and to highlight the need for further studies to address remaining concerns.
METHODS
Study design and study population
This was a local observational study consisting of retrospective chart review of all children and adults with HA diagnosed and treated with emicizumab and other treatment options at Sheikh Khalifa Medical City (SKMC) and Tawam Hospital in the United Arab Emirates (UAE) between January 2019 and December 2023. First, eligible persons were identified. Then, data were retrospectively retrieved from the medical records, and reported on an Excel datasheet for data analysis.
Individuals diagnosed with severe HA and followed by the haematologists at the participating centres, and treated for HA with emicizumab and other treatment options were eligible for inclusion. A documented waiver for the informed consent document was approved by local regulatory authorities and the Institutional Review Board (IRB). Persons without any assessable medical records, persons with moderate or mild HA, or persons lost to follow-up were excluded.
Endpoints
The primary endpoint was annualised bleeding rate (ABR) from baseline (first day of receiving treatment) to Month 12 among all eligible persons treated with prophylactic emicizumab and other treatment options. ABR was defined as the count of all reported bleeding events divided by the number of months in the reporting time window (8 weeks to 12 months) and multiplied by 12.
Secondary endpoints included the presence of FVIII inhibitors, HRQoL at Month 12 after switching to emicizumab using the Haemophilia-Specific Quality of Life Index (Haemo-QoL questionnaire for children and adolescents[11] and Haem-A-QoL questionnaire for adults[12]), joint bleed episodes, need for rescue medication administration at Month 12 after switching to emicizumab, Emergency Department visits at Month 12 after switching to emicizumab, and the safety profile of emicizumab.
Data collection
The medical records of included persons were reviewed to extract the following data: demographics (age, gender), previous line of therapy before emicizumab, 105 presence of FVIII inhibitors, joint status/arthropathy after treatment initiation / switching from previous therapy to emicizumab (count of reported joint bleeding episodes, affected joints [knees, ankles, elbows, hip, wrist, and shoulder]), HRQoL using the Haemophilia-Specific Quality of Life Index (Haemo-QoL questionnaire for children and adolescents and Haem-A-QoL questionnaire for adults), rescue medication administration, Emergency Department visits, and safety profile of emicizumab.
Statistical analysis
All available medical records for children and adolescents diagnosed with HA during the study duration were included in this analysis. It was expected that approximately 30 persons would be included in the study at the participating sites.
Continuous variables were reported as mean and standard deviation when normally distributed, and the median and interquartile range when skewed. Categorical data were summarised as frequencies and percentages. The normality of the data was tested visually using Q_Q plots and statistically using the Kolmogorov–Smirnov test. Parametric statistical tests such as analysis of variance (ANOVA) test and Student’s t-test were performed to compare means between groups when parametric assumptions were met. Otherwise, the Kruskal-Wallis and Mann-Whitney U non-parametric tests were used if data showed a skewed distribution in at least one of the compared groups. Differences were regarded as statistically significant if the p-value was less than 0.05. The statistical analyses were performed using IBM SPSS software for Windows Release version 23.
Ethics
This study was approved by the relevant IRB of the two participating centres. A documented waiver for the informed consent/assent was used, as required by local regulatory authorities, and/or IEC/IRB. The study was conducted according to the principles of Good Pharmacoepidemiology Practice (GPP;[13]), Good Clinical Practice (GCP;[14]) and the ethical principles that guided development of the Declaration of Helsinki. Data were anonymised prior to analysis.
RESULTS
Demographic and baseline characteristics
All of the 30 included persons were males with a mean age of 16.8 ± 11.4 years. In total, 73.4% of these persons received a rescue medication before initiating emicizumab. Joint bleeding was the most common reported indication for rescue medication (n=20/22; 90.9%). The mean duration of emicizumab therapy was 11 ± 2.3 months (Table 1). Reasons for switching to emicizumab were: presence of inhibitors (n = 15; 50%), difficult vascular access (n = 6; 20%), and patient/family request for better compliance (n = 9; 30%).
Table 1.
Demographic and baseline clinical characteristics of the study population (N=30)
Clinical effectiveness of emicizumab
All of the clinical parameters statistically improved after emicizumab initiation (Table 2). The ABR decreased significantly from 2.9 ± 2.4 before emicizumab therapy to 0.0 ± 0.0 after therapy initiation (p < 0.001). The decrease in the ABR before and after initiating emicizumab was more significant in patients with prior presence of FVIII inhibitors (3.9 ± 2.7 versus 0) than in those without FVIII inhibitors (2.0 ± 1.9 versus 0) (Figure 1).
Figure 1.
Annual bleeding rate before and after emicizumab initiation (N=30)
*The change in annual bleeding rate after emicizumab initiation was statistically significant for both groups (p value <0.001).
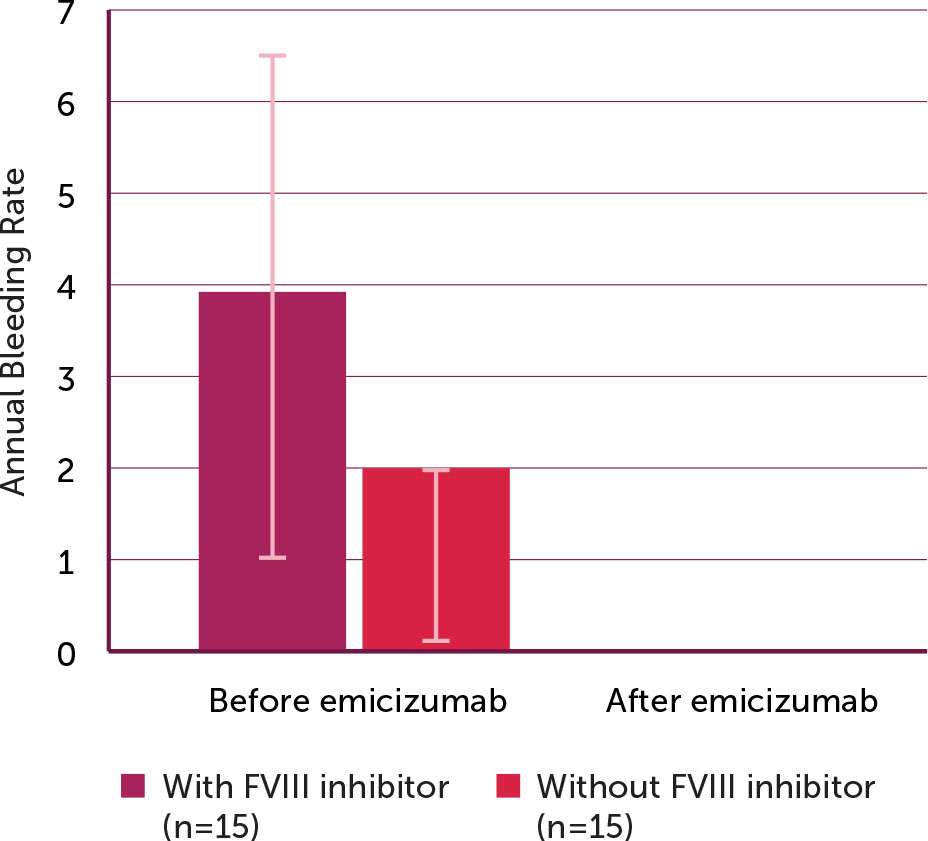
Table 2.
Clinical effectiveness of emicizumab (N=30)
| BEFORE EMICIZUMAB (MEAN - SD / N (%)) | AFTER EMICIZUMAB (MEAN - SD / N (%)) | P-VALUE* | |
|---|---|---|---|
| FVIII inhibitor presence | 15 (50%) | 1 (3.3%) | < 0.001 |
| Joint bleeding episodes | 2.3 ± 1.9 | 0.0 ± 0.0 | < 0.001 |
| ABR | 2.9 ± 2.4 | 0.0 ± 0.0 | < 0.001 |
| Requirement of rescue medication | 22 (73.3%) | 0.0 ± 0.0 | < 0.001 |
| Number of ED visits | 3.3 ± 2.8 | 0.0 ± 0.1 | < 0.001 |
| HRQoL total score | 78.0 ± 5.5 | 30.5 ± 3.7 | < 0.001 |
None of the study subjects required rescue medication during emicizumab therapy (p < 0.001). FVIII inhibitors significantly decreased after emicizumab therapy (p < 0.001). Joint bleeding events significantly disappeared after emicizumab therapy (2.3 ± 1.9 versus 0.0 ± 0.0; p < 0.001) as well as the number of ED visits (3.3 ± 2.8 versus 0.0 ± 0.1; p < 0.001). Similarly, HRQoL showed a significant improvement after emicizumab therapy (a score of < 40% indicates good HRQoL) (Figure 2).
DISCUSSION
The aim of our observational study was to review the clinical outcomes of emicizumab prophylaxis and the other treatment options in individuals with HA at two tertiary centres in the UAE. The reported ABR substantially decreased from 2.9 ± 2.4 to 0.0 ± 0.0 (p < 0.001). The long-term efficacy of emicizumab was demonstrated in a pooled analysis of the HAVEN 1–4 clinical trials, showing that ABR continued to decrease with ongoing emicizumab treatment and were maintained at < 1 after 24 weeks of prophylaxis[15]. Improved ABR was regardless of age, FVIII inhibitor status, or dosing regimen. A similar trend was reported in several observational real-world studies regardless of the FVIII inhibitor status[16, 17, 18].
Several clinical trials have shown that emicizumab is effective in people with HA with and without FVIII inhibitors[15]. Furthermore, emicizumab therapy was associated with a significant decrease in FVIII inhibitors level, a trend demonstrated in our study and other clinical and real-world studies[5,17,19]. The structure of emicizumab does not induce or enhance the development of FVIII inhibitors owing to the lack of sequence homology with FVIII[20]. Of note, the minimised risk of FVIII inhibitor development is a benefit for people on emicizumab prophylaxis even though they continue to be exposed to FVIII concentrates to treat episodes of breakthrough bleeds; this exposure was significantly reduced in our cohort during emicizumab therapy.
Haemarthrosis is the most common site for bleeding, particularly in ambulatory persons[21]. Moreover, joint bleeds are among the most clinically significant bleeds as they can cause arthropathy on the long term[22]. In our cohort, mean joint bleeds decreased significantly after emicizumab therapy. This was in line with the findings of a pooled analysis the HAVEN 1–4 trials; long-term emicizumab prophylaxis showed that 74.1% of participants had zero all bleeds (treated and untreated), 97.6% had ≤ 3 all bleeds, 94.1% reported no treated target joint bleeds, and bleeding into target joints decreased substantially[15]. Similar outcomes have been reported in several real-world studies; joint bleeds and bleeding events improved after emicizumab initiation[16,17,23].
HRQoL is an important aspect of the management of HA. Frequent bleeding and the development of target joints have been found to be associated with impaired HRQoL[24,25]. HRQoL can also be affected by treatment burden, including hospital visits, intravenous route of FVIII, and frequent and time-consuming FVIII administration[26]. Consistent with the improved clinical outcomes and good tolerability of emicizumab in our study, HRQoL significantly improved after emicizumab prophylaxis. This was in line with several clinical studies[9,23,27,28,29]. In the HAVEN trials, the Physical Health domain showed rapid and sustained improvement. Different factors may have collectively contributed to HRQoL improvement: reduced rate of bleeding events, increasing target joint resolution, good drug tolerability and low rate of adverse events, subcutaneous route of administration of emicizumab. It is noteworthy that only one person reported skin rash at the site of injection. Furthermore, significant reduction in the requirement of rescue medications and the subsequent reduction of Emergency Department visits may also have contributed to improved HRQoL.
Strengths and limitations
One strength of our study is that it was conducted in two different tertiary hospitals and was able to directly compare individual clinical outcomes before and after starting emicizumab. However, data interpretation in our study is limited by several factors, including the descriptive nature of the analyses, constrained by recruitment challenges. Furthermore, the small population size with inter- and intra-person variability during the study period limits the ability to further analyse by potential confounders. In addition, the study included a single arm which precludes comparison between emicizumab with another form of prophylaxis. Moreover, reliance on chart review may introduced some bias in terms of missing or misreported data. While bleeding events that were not documented in the medical record or reported by persons may not have been documented, the likelihood of this would be similar before and after emicizumab therapy.
CONCLUSION
The results of our real-world study demonstrated that emicizumab prophylaxis is effective, safe, and well-tolerated in persons with HA with and without FVIII inhibitors. Emicizumab was associated with improved clinical outcomes in terms of reduced reported bleeding events, joint bleed episodes, use of rescue medications, and FVIII inhibitor levels. Given the detrimental impact of HA on HRQoL, our results also suggested that emicizumab prophylaxis improved HRQoL.
