The EHC Think Tank Workstream on Access Equity has identified a need for interventions for challenges associated with patient journeys and pathways, behaviour and mindset, and budgets and resources to ensure equity of access to healthcare services in rare diseases

Advances in technologies and treatments are raising expectations for life-changing developments in the care of severe rare and chronic diseases [1]. However, resource constraints and priority funding for more common conditions, including cancer and heart disease, can result in inequity of access to treatment and care, and the risk that people with rare conditions miss out. In the case of haemophilia, for example, there is uncertainty about how the implementation of the European Union (EU) regulation on health technology assessment (HTA) (2021/2282) [2] will affect access to novel therapies, while in low- and middle-income countries (LMIC), clinicians struggle to access older therapies [3,4].
Against this background, the European Haemophilia Consortium (EHC) Think Tank has established a Workstream on Access Equity, with the aim of identifying and addressing the lack of access to treatment and care, and how to achieve access equity across patient populations, as well as within local, regional and national boundaries. ‘Access’ refers to the ability to benefit from any given aspect of treatment and care [5], while ‘equity’ refers to the fair and impartial provision of access to care across and within countries, at both community and individual levels [6].
At the first virtual workshop of the Access Equity Workstream on 3 February 2023, participants representing a wide range of stakeholders, including healthcare providers, patient groups, research, and industry, shared their perspectives in order to identify the key challenges to achieving access equity. Seven challenges were identified:
Patient journey and pathways
Behavioural change, mindsets and incentives
Budget and resources
Creating transparency
Upcoming supply issues for therapies
Uncertainty regarding regulations
Information, education and health literacy.
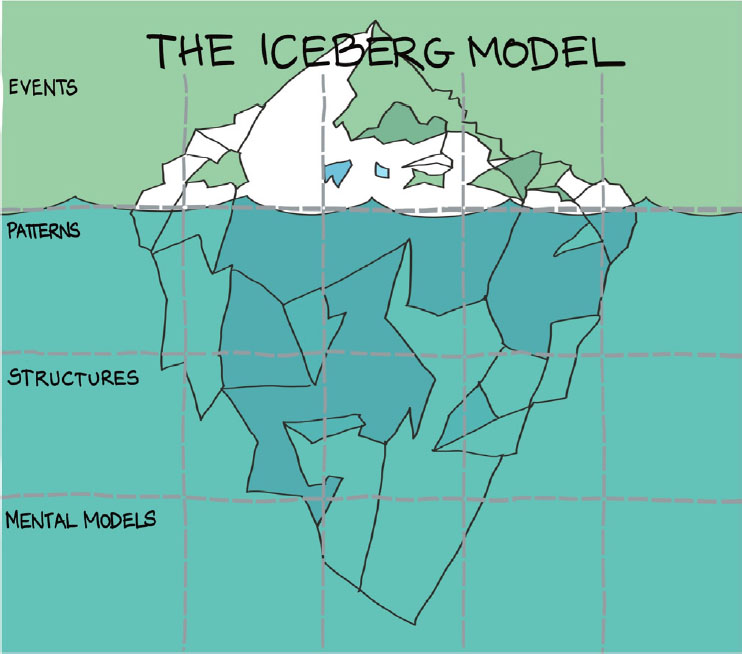
It was agreed to prioritise challenges 1, 2 and 3 for further discussion. The Iceberg Model was used to identify the factors (events, patterns, structures, and mental models) that should be the focus of future discussions about potential interventions (Figure 1) [7,8].
Figure 1.
Iceberg Model template used to identify events, patterns, structures and mental models in challenges for access equity. Image: Ia Brix Ohmann / Overlap (https://www.overlap.dk/english)
PATIENT JOURNEY AND PATHWAYS
1.
Rare and chronic conditions can involve complex patient journeys and pathways. The patient journey is generally understood as an individual’s experience and interactions through various settings in the healthcare system over time [9]. Patient pathways have been defined similarly, focused on decision-making and the organisation of care processes [10]. For the purposes of this part of the Workstream discussion, the ‘patient journey’ is defined as the individual’s lifetime experience of living with their condition, and the ‘patient pathway’ refers to a specific intervention that forms part of that journey.
For people living with rare disease, patient journeys and pathways encompass multiple potential issues with regard to healthcare and social support, both for themselves and for their families, carers and friends. Before considering these issues and how they can be addressed, clinicians need to be confident of the quality of the pathways that patients will encounter during their journey. The European Commission established the virtual European Reference Networks [11] to facilitate discussion on complex or rare diseases and to agree and implement best practice across the EU, but some countries do not have the resources to follow this guidance.
In striving for equity of access to optimal pathways during the patient journey, clinicians need to be sure that patients are provided with the best possible care. Each patient is different and will have different needs for care and support, information and training [12,13]; pathways and access equity therefore need to allow for flexibility as well as optimisation of care.
The first event in the patient journey is likely to be diagnosis but there is considerable national, regional and even local variation in access to primary care and referral for specialist consultation. Ease of referral and delays in referral to the correct specialist, local availability of expertise, waiting times, and affordability will all impact access equity. There is also gender bias in the healthcare system in general [14]. In the case of bleeding disorders, women and girls are typically diagnosed at a later age than men and boys due to lack of awareness that bleeding disorders affect both sexes [15,16].
There is wide variability in access to prenatal and newborn screening due to differences in healthcare systems, available technology and cultural acceptance [17]. Alongside this, there is a lack of agreement on what should be screened for (and why), which may impede the development of services such as genetic testing and counselling for families with known inherited diseases [18]. In terms of mental models, the ethics of screening has to be considered and the implications for patients and families, especially if no treatment is available [19].
Equity of access to treatment is a major factor impacting patient journeys and pathways in rare conditions. Access to specialists with prescribing expertise may be limited, while regulatory approval and availability of specific therapies varies across and within countries. Affordability is a major issue, especially for newer agents [20] and in countries where health spending is a lower-than-average proportion of gross domestic product (GDP). Pricing and reimbursement severely affect access equity. Gene therapy presents significant progress in the treatment of some rare disorders, but there will be debate about sharing limited budgets to treat small numbers of people with such an expensive therapy, compared to treating larger numbers of patients with common diseases with cheaper drugs [20,21,22]. ‘Social solidarity’ is seen in many healthcare systems across Europe, whereby all individuals are entitled to equal access to a reasonable standard of care regardless of their ability to pay [23]. Solidarity towards people with genetic diseases is important to ensure equal access to screening, diagnosis and treatment, and patients need to be self-motivated to make the best use of what is available to them and, where possible, to advocate for improvements [24].
BEHAVIOURAL CHANGE, MINDSET AND INCENTIVES
2.
When considering the contribution of behaviour, mindset and incentives to access equity in rare diseases, it is important to look across all stakeholders, notably healthcare providers, policymakers, industry and the patient community. Faced with an ageing population with associated comorbidities and complexities, and with growing healthcare demand, providers and policymakers may adopt a ‘savings mindset’ and freeze budgets [25], but this is counterproductive for access equity. Short-termism may mean restricted allocation of funding for innovative and preventive therapies, and resource allocation may occur without detailed knowledge of the issues [26]. A behavioural focus on reducing costs while maintaining the current system may result in ‘quick fix’ decisions being implemented without adequate understanding of potentially adverse long-term implications [25]. This is unhelpful and may be detrimental to access equity.
The industry mindset is focused on the need to reward investors [27]. The need to generate income to fund future research investment has been a longstanding and understandable basis for setting drug prices. However, with a move towards targeted medicines and incentives for the development of orphan products [28,29], it is questionable whether pharmaceutical research is as high risk (and expensive) as previously. Escalating prices of medicines are unsustainable, and there needs to be a change in mindset from the current transactional to an outcomes-based approach, with greater transparency about real costs in order to generate trust.
The methodology for assessing the value of treatments to society is quite rudimentary. While the development of EQ-5D as a standardised measure of health-related quality of life has been useful [30], the move towards bespoke adaptations for specific diseases has ‘diluted’ its effectiveness for comparing the value of different therapies across disease areas. This can adversely affect the identification of opportunities for promoting access equity. Within the patient community, there is an understandable behavioural tendency to focus on specific diseases instead of the wider implications for the provision of healthcare services and for society. Patients may feel that the system is ‘against their disease’ and that they are excluded [31,32]. There is often a general lack of understanding about how the system works and the priorities and mindset of other stakeholders, together with a misalignment of personal, collective, organisational and national needs.
BUDGETS AND RESOURCES
3.
It is difficult, if not impossible, to discuss access equity without talking about budgets and resources. Over the coming years, there will be a considerable conflict between the introduction of expensive new drugs for a broad range of diseases and the sustainability of healthcare budgets [33,34].
Delayed access to drugs is problematic, especially in rare diseases [35]. Proving efficacy in small clinical trials in rare diseases can be challenging, so marketing authorisation may be conditional. However, health technology assessment (HTA) is subject to cost-effectiveness prioritisation tools that do not take account of data from small populations, leading to cost-effectiveness ratios that exceed accepted thresholds [20,36]. As a result, HTA bodies and payers consistently restrict treatments to subgroups of patients within an approved indication. There is a mindset that there is not enough budget to treat all patients, and the focus is on immediate costs instead of long-term investment to generate future savings.
Given the difficulties of achieving reimbursement for expensive new medicines, pharmaceutical companies may choose to limit launches to countries where there is least pressure on budgets and resource allocation is less challenging. Benchmarking international pricing raises multiple issues [34,37]: even across Europe, prices that are acceptable in a major, well-resourced country may be too high for smaller countries with limited budgets. Pharmaceutical companies are likely to be reluctant to set a relatively low reference price in one country as this will affect pricing in other markets [29].
A lack of centralisation in Europe for key elements needed for access equity – such as negotiating, funding, procurement and service delivery – adds to the difficulties facing national governments. A mindset of reluctance to give up national control over the introduction and delivery of expensive new therapies, including gene therapy, may impede cross border collaboration. However, in countries with small numbers of patients and limited expertise and resources, providing patients with treatment in other, more well-resourced countries may be the most realistic option [38,39]. The European Rare2030 Foresight Study recommends the facilitation of integrated care provision for rare diseases through collaboration [40].
CONCLUSIONS
The challenges associated with access equity are broad in scope, and workstream participants recognised the deep-rooted and often contrasting mindsets of stakeholders that inform and drive events. While focusing on challenges around Patient journey and pathways, Behaviour, mindset and incentives, and Budgets and resources, elements of other challenges that had been identified also formed part of the discussion. Future workshops will need to address the challenges of achieving access equity at key stages of the patient journey, including diagnosis, treatment and screening. For the behavioural changes needed to achieve access equity, the misalignment of personal, collective, organisational and national needs will need to be addressed alongside systems that are currently not fit for purpose. Tackling issues around service centralisation and cross-border cooperation for delivery of gene therapy will be key to addressing one of the major budgetary and resource challenges to access equity.
ACKNOWLEDGEMENTS
This paper became feasible through the valuable contribution of the members of the EHC Think Tank Workstream on Access Equity.
The authors have advised no interests that might be perceived as posing a conflict or bias.
This paper does not contain any studies involving human participants or animals performed by any of the authors.
THE EHC THINK TANK
The European Haemophilia Consortium (EHC) Think Tank was launched in June 2021 Building on existing advocacy activities, the initiative brings together a broad group of stakeholders to engage with key thematic areas or workstreams identified as priority areas for ‘systems change’ within European healthcare systems [41]. The EHC Think Tanks seeks to mobilise the agency and purpose of all stakeholders in the healthcare system to collectively design and champion potential solutions to existing problems.
Workstream members are invited based on their expertise and potential for constructive engagement, including patient and industry perspectives alongside a balance of healthcare professional, academic, regulatory, governmental and geographical representation. All workstream activities are held under the Chatham House rule to enable inclusive and open discussion: participants are free to use the information received, but neither the identity nor the affiliation of the speakers, nor that of any other participant, may be revealed [42]. Each is projectmanaged from within its individual membership. Members set their own agendas, timelines, and targeted outputs, with operational, logistical, methodological and facilitation support from EHC staff and Think Tank practitioners. While concrete outcomes and results will vary across workstreams, they are likely to include (but not be limited to) manuscripts, consensus-based guidelines, monographs, white papers, and so on.
Since the Think Tank’s inaugural workstream meetings in 2021, the following key topic areas have been the subject of ongoing discussion:
2023 sees the introduction of two new workstreams: