Gene therapy offers great potential for people with haemophilia [1,2]. Haemophilia is an ideal target for gene therapy, as it is caused by a single mutation and has a well-characterised genetic profile [2,3]. In addition, even a small increase in factor activity can alter the disease phenotype [1]. Several gene therapies are currently being investigated for haemophilia A and B, with promising efficacy demonstrated to date, particularly those using adeno-associated vectors (AAV) [4]. Clinical trials of investigational products have delivered sustained and clinically relevant benefits, including reduction in annualised bleeding rate and cessation of prophylactic factor use [5,6,7,8,9,10,11,12,13,14].
A qualitative exploration of the experiences of people with haemophilia receiving gene therapy as part of clinical trials highlights the role of health care professionals as a key trusted source of information, and points to the pivotal role of the haemophilia nurse.

Whilst the efficacy results in clinical trials look encouraging, we must be mindful that gene therapy presents significant challenges for people with haemophilia (PwH). There is a need to investigate the perceptions of PwH around gene therapy so that we can better understand individual motivations and expectations, and to help us support people with haemophilia at key steps in their journey. In haemophilia, as in many chronic diseases, the multidisciplinary team plays an invaluable role [15,16]. As we enter a new era of gene therapy, nurses within the team will have a critical role in counselling, education and support.
The results of telephone interviews in three people with haemophilia B who have undergone treatment in the AMT-060 trial have recently been published [17]. This paper aims to further enhance the understanding of perspectives around gene therapy, including concerns and motivations, through testimonials from PwH receiving a number of different investigational gene therapy products at a single treatment centre – the Royal Free Hospital in London, UK.
METHOD
In December 2019, participants were identified from among the 21 PwH treated to date with an investigational gene therapy product for haemophilia A or haemophilia B at the Royal Free Hospital. The choice of participants was based on availability rather than any pre-selected clinical criteria.
From December 2019 to January 2020, structured, qualitative, one-to-one interviews were conducted by a clinical nurse specialist involved in day-to-day care in the clinic and known to the people taking part. Participants were offered the choice of an in-person or telephone interview. The interview questions were provided to the participants ahead of time. All face-to-face interviews were completed within a scheduled clinic visit; none of the participants was asked to attend specifically for the purposes of the interview. No time limit was set for the interviews – participants were able to speak for as much or as little as they wanted.
The interviews consisted of a series of 13 open-ended questions about the participants’ gene therapy experience (Figure 1). They were free to give as much or as little information as they chose, and the interviewer did not press or probe for additional or specific answers. For the most part, no additional prompts were used; question 10, on the follow-up regimen, included prompts on alcohol consumption and family planning.
The interviews were audio recorded and later transcribed verbatim. From the transcripts, the responses were thematically analysed using a latent inductive approach [18]. This hermeneutic phenomenological method allowed us to examine the participants’ lived experience [19], their perceptions about their gene therapy journey, their motivations and fears, and their satisfaction with their decision to trial the treatment.
We were aware that the response to gene therapy would be an important factor in determining the patient experience of the treatment. However, as the patients are still within the study period and the results have not yet been published, this was not included as part of this study. We did not collect any data on efficacy outcomes for any particular investigational gene therapy product and did not attempt to stratify results by which product each patient had received.
Ethics and consent
The Royal Free Hospital operates under the UK National Health Service. An informal ethics opinion was sought, and the UK Medical Research Council decision tool was consulted [20]. As our survey did not involve randomising patients, changing treatment, or looking for generalisable findings, it was confirmed that the planned interview series was not considered to be research, and did not require ethics review [20].
All participants had given their written informed consent for the clinical trial program. In addition, they were required to give their verbal informed consent to be included in the interview process, and for their anonymised data to be used in a symposium presentation at the 2020 EAHAD Congress and in this subsequent publication.
RESULTS
Seven PwH who had received an investigational gene therapy infusion as part of a clinical trial at the Royal Free Hospital were invited to be interviewed to discuss their experience of the journey, and six agreed (anonymised here as R1–R6). The person who declined did so for logistical reasons. This interview group represents almost one third of the 21 patients treated to date with an investigational gene therapy product for haemophilia at the Royal Free Hospital. Four interviews were conducted in person, and two took place by telephone. On average, the interviews lasted for 10–15 minutes.
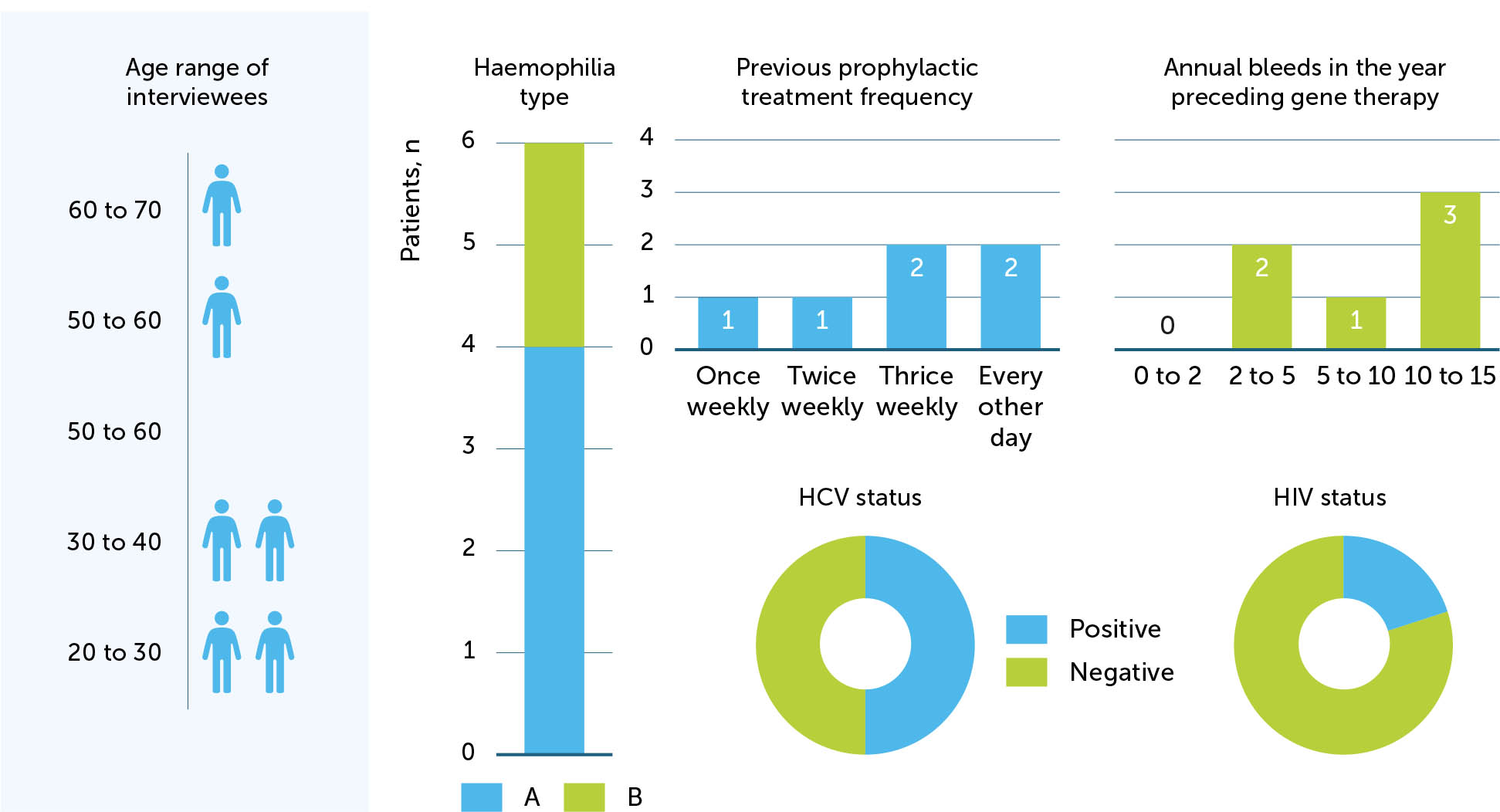
Four participants had haemophilia A and two had haemophilia B. Four participants were under the age of 40. The mean time out from their gene therapy infusion was 10 months (range 4–18 months). Three were HCV-positive and one was both HCV- and HIV-positive. Two participants had previously undergone joint replacement surgery. Prior to receiving the gene therapy infusion, all participants had been on prophylaxis, two with extended half-life products, and the range of annual bleeds reported in the year immediately preceding was 2–15. Participant demographics are shown in Figure 2.
Pre-infusion
Main questions and concerns prior to receiving gene therapy
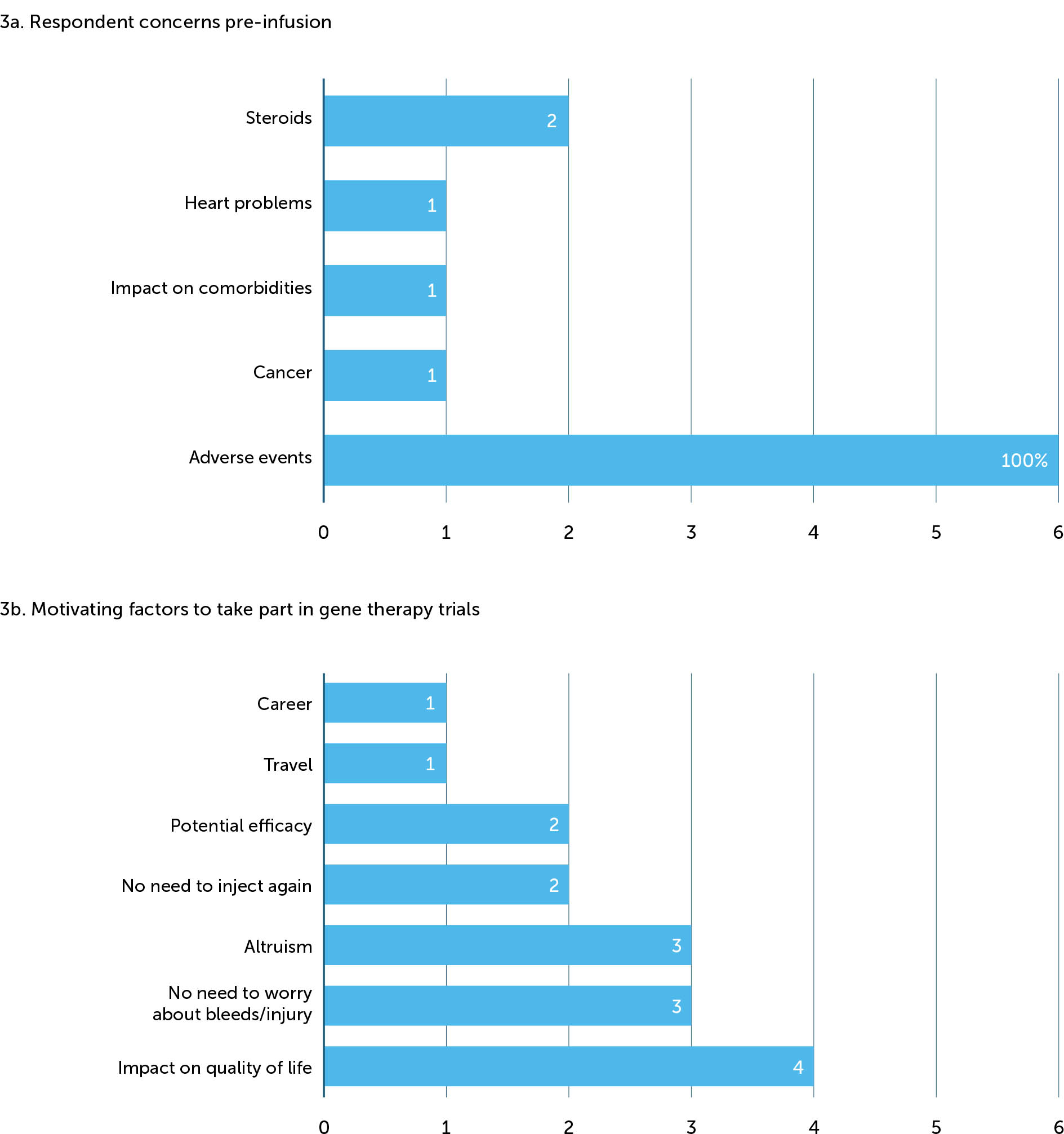
Perhaps unsurprisingly, the main concern that people with haemophilia had prior to receiving gene therapy centred on potential side effects (Figure 3a). Of our six interviewees, all noted that pre-infusion they had been worried about some of the possible side effects of therapy. This appeared to form an important part of their decision-making process, although their concerns were not strong enough to prevent them being dosed. Specifically, one respondent mentioned cancer, one the impact on existing HIV, one referenced heart problems, and two remembered being afraid of needing to use steroids. One interviewee also mentioned some fears around the viral vector but had been reassured by staff in the centre. Other concerns pre-infusion related to the predicted long-term efficacy of gene therapy (3/6); only one participant remembered having questions about the patient experience of taking a gene therapy product.
“[I wanted to know about] the experience of other patients, I wanted to know about potential side-effects and benefits – everything really.” (R2)
“For me, [the] major concern was that I wouldn’t be able to have another gene therapy in the future if this trial one didn’t work. And then about side-effects – not the inconsequential ones, but more severe ones like heart problems. I was keen to take part, but worried about the timing.” (R4)
“Before I did it, it took a year to decide. I saw a patient advocate. I had made my mind up I was going to do it. But the advocate totally changed my mind – he went through a list of horrible potential occurrences that may or may not happen. Afterwards I spoke to my mum, dad, sisters, partner, and my best mate. It was my mate who turned me around again and said to give it a go… the patient advocate scared me, but I can’t remember half of it now.” (R5)
Key factors that encourage people to consider having gene therapy
The most important factor for participants was the impact that gene therapy would have on their lifestyle or quality of life (Figure 3b). Three highlighted the importance of not needing to worry about bleeding or injuries anymore, two mentioned never needing to inject again, and two referred to the potential efficacy. One mentioned travel as a key factor in their decision to have gene therapy, one stated that being able to put a stop to further joint damage was attractive, and one made reference to a career path that would open up to him if he was no longer classed as having haemophilia.
“Key factors were overall improvement of day-to-day life; not having to worry as much about hurting myself. Being able to do more. A general better quality of life. I guess additionally, from a more empathetic side, it could help a lot of other people like me. Selfish and an unselfish reason. But both made sense.” (R5)
“If you don’t try these things, no one benefits. I was confident it would work, and if it made me a mild phenotype that would be an advantage to me and my lifestyle, just by no longer needing prophylaxis.” (R3)
“The main thing was the idea of not having to be on prophylaxis or have the worry of carrying treatment or injuring myself. But knowing what damage has already been done, to put a potential stop on that – even temporary – that's the main thing for me.” (R2)
Sources of information prior to treatment
The main source of information was the treatment centre and the health care professionals working there. Only two respondents had proactively looked elsewhere for information to support their decision – both had researched online, and one had talked to people who had previously had gene therapy. Although our sample is small, we were interested to see if there was a difference in how people in different age groups behaved. Those in the youngest and oldest age groups (20–30 and 60–70) were content to rely entirely on the centre for information; those who searched for additional information fell into the two middle categories (30–40 and 50–60 years of age). Of the two interviewees who had looked for information online, one stated that it was hard to find the right information, and that a lot of what was available was not suitable for patients.
“[I had] lots of information from [the] centre, previous patients, and did some research online – but a lot of the information online is aimed at health care professionals, so it wasn’t the right thing for me. Reading online wasn’t very useful, and it was hard to find relevant information.” (R2)
“I had a number of discussions with doctors; I had more meetings and discussions than for any other trial or treatment in the past.” (R1)
“Centre information, nothing from outside. Didn’t speak to any other patients.” (R6)
First awareness of gene therapy
All respondents said they had heard about gene therapy from the treatment centre, though one indicated they had also seen news articles and were aware of the technology from the media.
The role of patient support networks
Four participants had not spoken to any other patients about their experience of gene therapy prior to their own infusion. One had used a group on social media (Facebook) that they had found helpful, although they did not elaborate on how they used it, nor whether it was geared solely towards discussing gene therapy or was a general haemophilia community group. Although not directly addressed in their responses to this question, participant R5 mentioned talking to a patient advocate.
Family considerations
The main considerations for family members were potential risks and side-effects. Two respondents said their families were excited for them to take part, and most (5/6) responses indicated that families were supportive of the decision.
“My wife was concerned about some of the risks.” (R1)
“[My family] were excited at the prospect of a potential cure and not having to inject. But then they were worried that the side effects were unknown, and potentially big. They are not so connected to the disease as me, and the thought something could go wrong 10 years down the line was scary for them.” (R2)
“My brother was keen for me to go for it. My wife was encouraging as long as I was confident in it.” (R3)
“My parents were excited for me. It was overwhelming for them – they needed as much counselling as I did. My partner came to the centre with me to discuss it. Once I made my mind up that I wanted to do it, it couldn’t come quick enough.” (R4)
“My mum didn’t really question it, she said to go for it. My dad had a lot more concerns. He was very dubious about it. Ultimately the decision lay with me.” (R5)
“[My family] wanted to me do it. There were concerns about effect on liver/cancer, but overall they weren’t worried.” (R6)
Peri-infusion
Concerns and questions about infusion day
When considering the responses to this question we should bear in mind that the interviews were done 4–18 months after the fact; however, none of the participants expressed any concerns about the infusion day itself. All said they had no real concerns on the day and remembered feeling positive and hopeful about what the results might be. Two indicated there had been a lot of build up to the day of infusion, and that they had been ‘psyching themselves up’ to deal with it, but most expressed no anxiety, and none indicated that they had any last-minute questions or concerns. However, one recalled worrying about whether the infusion would work and being unsure how he would react mentally. Another remembered being surprised that the infusion would take an hour through a drip – they had expected a single injection.
“No real concerns; just wanted it over and done with. It seemed like a big build-up, it took months, but once it was done the decision had been made and there was no going back.” (R1)
“No real concerns. I remember thinking it would be life-changing. Tomorrow is a new day.” (R3)
“No real concerns. Prior to infusion I was psyching myself up. For me, it would have been better to have some counselling beforehand to prepare me for the life-changing nature of the treatment. The results were so dramatic. The hardest part prior to infusion was worrying if it would work, and how I would mentally react.” (R4)
“No – I didn’t know I would have to sit there for an hour being drip-fed – I thought it would be one quick injection. Obviously it was fine, but I didn’t know how long it was going to go on for.” (R5)
Patient experience of infusion
All participants found the infusion to be simple or uneventful. Words typically used to describe the experience of the infusion itself were anti-climax, fine, simple, boring, and uneventful. However, as mentioned above, one person had not been prepared for what would happen on the day and was not expecting to have an infusion, or for it to last as long as an hour, and another had indicated that when it came to it he did not feel mentally prepared.
“The infusion was a bit of an anti-climax – just 1 hour and then I could go. It was simpler than I had been expecting.” (R1)
“Fine. I did feel at one point just as the infusion was being prepared that there was no turning back. There were quite a lot of people around. I could have used more mental preparation.” (R4)
“It went fine. Oh, is that it… fine. It was quite boring sitting around all day doing nothing. I wasn’t scared or nervous. It was too late by then anyway, so you have to suck it up and get on with it. But by that point I had made up my mind. I wasn’t apprehensive.” (R5)
Post-infusion
Adjustment to the follow-up schedule post-infusion
In the initial follow-up period, four participants found the frequency of hospital follow-up appointments difficult or inconvenient, but not so much that they would be put off from doing it again. Time and travel were the main issues noted. One indicated that he was actively reassured by the visits and happy to keep coming to the centre. Two required steroid treatment that caused side-effects, and both used the word struggle when talking about the follow-up schedule. The general consensus was that after the initial period the follow-up was easier, and two noted that their employers were flexible with regards time off for hospital appointments.
“I have done so many trials, for me it's not an issue. But my work is flexible. The hospital visits are a pain, but not enough to put me off.” (R1) “I was mentally prepared for it. Getting to the hospital is a long journey for me. But I was prepared. It is a pain. Sometimes I want to miss the odd day.” (R2)
“It was arduous because of the distance from the centre, but I was lucky to be involved and happy to do it. It's only the first six months that are quite hard going, then it eases off.” (R3)
“I have a very supportive employer, and it was reassuring to come into the centre to see people I have known for many years to closely monitor me. I was happy to keep coming up and giving samples. For me, it wasn’t a problem. I was recompensed for the travel, although some people may need that on a monthly basis – mine was after six months.” (R4)
“Naturally. Easily. Initially post-infusion it was really difficult as I had to take a lot of steroids, and I really struggled with that. Lack of sleep, the spots on my back, my face puffed out and was sore. I was agitated, mood swings, and was constantly eating. And I was signed off work for a couple of weeks. But once I was off the steroids everything was breezy.” (R5)
“It was only right at the beginning it was difficult as I was going to hospital so often – that was a struggle. When I was on steroids for longer than planned my face got big and I didn’t like that.” (R6)
Follow-up regimen
The question about the follow-up regimen included prompts on alcohol consumption or family planning. Four participants had no problem in abstaining from alcohol for the specified period. However, two people in the two younger age categories (20–30 and 30–40 years) stated that they had found it difficult and that it had affected their social lives. Only one interviewee explicitly mentioned that the semen samples had been a problem, although with regards to family planning two men in the two middle age groups (30–40 and 50–60) had found it difficult to use a condom and thought it had put pressure on their relationships. One mentioned the potential stigma associated with a risk of passing a virus on to a partner.
“I didn’t drink or go out for three or four months, which does affect your social life. But other than that, there were no issues. Weight gain was a bit of an issue. Was not planning a family anyway. The semen samples were a pain to do actually.” (R1)
“No real concerns. Little bit concerning when they found three to four months after that CD4 had dropped back again, so had to take an inhaler once a month. That was a little unpleasant towards the end of the nebuliser. But that was just three visits.” (R3)
“I wasn’t thinking of having children anyway, but having to wear a condom wasn’t great, but partner was supportive. It can put extra pressure on a relationship. I didn’t want to be in a position where people would treat me differently because they thought I might pass on a virus to them. For alcohol, I very rarely have it anyway, so no problem.” (R4)
“Alcohol – I love a drink, but that turned out to be fine. You’ve got to do it, and it's not the effect on you, it's for the study, so I knew that I couldn’t drink or it's not fair on the people doing all that hard work. But after three months I was straight down the pub. Family planning wasn’t ideal, but again it is what it is. That potential effect could have been really bad.” (R5)
Impact of gene therapy on daily life
When asked about the impact of gene therapy on daily life, the first thing that came to mind for three interviewees was not having to inject anymore – or not having to think about injecting. Travel was mentioned by two participants, and one described the transformative impact on his work and social life. Two gave anecdotal accounts of accidents they had experienced, which previously would have required treatment, but which resolved as they would for a person without haemophilia. One participant summed it up by saying he had “to learn to live without haemophilia”. The majority (5/6) expressed positive feelings, using words such as amazing, incredible, transformed, appreciation, gratitude and happiness – especially those who were further out from their infusion date.
“I don’t inject anymore. I’m less concerned about bleeding. In fact, I‘ve forgotten all about it. It's amazing how quickly you can adapt. I am still a bit anxious about my arthritis creeping in, and it may take a while to stop worrying about bleeds. It changes everything – now when we go on holiday I don’t have to fill half a bag with factor. You have to learn to live without haemophilia. We had planned everything around my disease before, everything else is secondary.” (R1)
“I am still kind of ongoing with it… not having to inject is a huge difference. I will probably have a better idea of this in six months’ time. The hospital appointments and steroids have made things a bit unpredictable. I have had some worse side-effects than other people.” (R2)
“It's unbelievable. Don’t have to think about injections. I don’t carry treatment with me if I’m local, although I do still take some if I travel. It's not a life without care, as I still try and avoid injury, but that probably won’t ever go away. A while ago I fell over badly and was convinced I would be covered in bruises, but I just had one small lump and no bruises anywhere. That was a big change. Before I would have been badly injured and have needed a lot of treatment. Life is considerably easier and more relaxed.” (R3)
“It's amazing. It has transformed my work and social life. I was always concerned about not being able to do my job because of bleeds or injury, but that's all gone now. Outside work, it's still hard to come to terms with it… it's incredible, and hard to put into words. It has transformed my life.” (R4)
“Hard to answer… even when I was still injecting regularly, I would forget I had it. So that even more – but sometimes [I] have a defining moment where I sit down and think it's amazing. I get massive waves of appreciation, gratitude and happiness. I feel very lucky. By doing this... if and when the time comes [that it stops working] we will be better equipped to come up with another solution.” (R5)
“It's a lot better. I never have to think about it or worry. I have had no injuries for over a year and half – including when I smashed my finger in a door, and would normally need an injection, but it swelled up like a normal person's would and then went down.” (R6)
Family perceptions after gene therapy
Four interviewees described their family perceptions now as excited, happy or overwhelmed; conversely, two felt that their families had forgotten about it. One participant talked about the possibilities that have opened up for his family as a result of his having had gene therapy, including being able to move abroad, which was not an option before.
“They have forgotten about it now too. It's me who it affects the most. For my wife, it does change how I feel about my future, and we’ve talked about moving abroad now which we couldn’t have done before.” (R1)
“They are okay with the idea now. There is no turning back. It's almost quite exciting for them now; they like seeing my weekly update on levels to see where it's going.” (R2)
“They don’t comment much on it… I was quite independent and got on with my life, so I didn’t make a big deal about my haemophilia. So, from their point of view my life on low-dose prophylaxis without many bleeds probably doesn’t look that different to them. My cousin is over the moon that I’ve had it and it was successful.” (R3)
Perceptions on the unknowns of gene therapy
When asked about their perceptions on the unknowns of gene therapy, all respondents seemed pragmatic and open-minded about the future, stating that they felt the outcomes of trialling a gene therapy product were worth the potential risks.
“What will be will be. I can’t worry about these things. I’d like to think that's it now, but we don’t know. I could have let other people trial the gene therapy and 20 years down the line that would have been a major regret for me. If I lose expression, I go back on factor – there are still alternatives.” (R1)
“It's hard to say at the moment. I don’t have much perspective on the past three months. There are so many unknowns. I don’t worry about it affecting me. I worry about it affecting those around me if side effects develop that aren’t contained to me. If other people's lives are affected that would be worse.” (R2)
“I think there aren’t many unknowns left… most problems and side effects have been seen. My perspective, it's worth it for the end result.” (R3)
“Currently I am a bit worried I keep thinking my heart is racing – but is that anything to do with it? We don’t know yet. But for me it's worth the risk. The effect on my daily life outweighs the potential risk down the line.” (R4)
“We’ve all got to go one day… you only have to tell us this stuff [about side effects] just in case. You wouldn’t be doing this to us if the chances were even 1%. But stuff can happen regardless of gene therapy. So, I tend not to think about it – otherwise, none of us would go outside.” (R5)
“Not anymore. I don’t really think about it. What will be will be.” (R6)
DISCUSSION
Compared to our colleagues in Germany, our interview group was a much wider age range, with higher rates of bleeding reported in the year prior to treatment, and represented more than one investigational product – yet some of our findings are very similar, especially around people reporting that a key advantage to life after gene therapy is not having to worry about injection[17]. When asked about their feelings about the future, most of our interviewees expressed hopeful, optimistic, yet pragmatic and open-minded views. Up to 18 months post-infusion, none of our interviewees regret their choice to take part in the trials of investigational gene therapy products for haemophilia. When thematically analysing the responses, we concluded that altruism was one of the driving forces in decision making, with half of our interviewees indicating that they felt taking part in gene therapy trials would give something back to the haemophilia community. Interestingly, altruistic reasoning was more common in the older age categories (>50 years), while the majority of younger people were more focused on lifestyle factors and benefits that could accrue to them personally. This may reflect the higher expectations of younger people with haemophilia and is an important facet to consider when introducing the idea of novel therapies. Our analysis also indicated a sense that siblings and parents of PwH are more open to gene therapy than spouses or partners. This may reflect the greater number of years that haemophilia has been a feature of their lives, or a heightened understanding in families who may have experienced the disease over many generations.
One issue that becomes clear from talking to PwH in this way is that we can do more to prepare gene therapy candidates ahead of their infusion. Although PwH are often well-informed – and there is a dynamic community – our selection of interviewees generally had low awareness of gene therapy prior to joining the trials. They were not motivated to do their own research and content to rely almost entirely on health care professionals at the treating centre for information, with four out of six participants making their decision to have gene therapy based solely on this. Similar findings have been reported elsewhere [21,22,23]. However confident we are in our own staff and our knowledge as a trial centre, when extrapolated this is an important finding. Results of a large-scale international survey published in 2020 [24] show that among physicians directly involved in haemophilia care, 35% lack the ability to explain the science of adeno-associated viral (AAV) gene therapy (which may become a more common concern for PwH considering gene therapy going forward, given heightened public awareness due to the coronavirus pandemic), and 40% are not comfortable answering patient questions about gene therapy for haemophilia based on clinical trial results to date. As gene therapy moves into the mainstream, there is a critical need for ongoing education programs for all staff involved in the care of PwH to ensure their preparedness to offer up-to-date information to candidates and their families. Undertaking gene therapy is a big decision that should not be taken lightly, and requires clear education and support, with consistent language [25]. We should not assume PwH will have access to materials that would enable them to learn about gene therapy and should be aware of the existence of misinformation in the public domain [23]. Patient advocates are already known to play a role in ensuring that PwH participating in gene therapy trials are informed and aware of the risks [26], reflecting the experience of one respondent who described being informed of ‘horrible potential occurrences’ and being scared of what he was told. Treating centres may also find it useful to build networks of experienced patients who are willing to offer support to new candidates on the journey.
At present in the UK, people with haemophilia are usually looked after in comprehensive care centres or haemophilia treatment centres [27,28]. Care is frequently delivered by a multidisciplinary team coordinated by specialist nurses [27], which for optimal care should include a haematologist, physiotherapist, orthopaedic practitioner, occupational therapist, psychologist, social workers, a counsellor and nurses [15,29]. The need to fully understand gene therapy is therefore not limited to haematologists and research teams but must extend to the full multidisciplinary team. Our interviews were conducted by a clinical nurse specialist involved in day-to-day care. Nurses in particular are critical and already play a central coordinating role in delivering comprehensive care for PwH and helping to promote adherence [16,27,28,30,31]. It is well known that nurses are able to foster good relationships with patients, and that patients are often more candid with nurses than they may be with doctors. Nurses can empower people to make the right choice for them. and offer ongoing support and counselling, in addition to playing a key role in comprehensive care [30]. One of the major benefits provided by nurses is education [31]. In the future, nurses will be critical in helping PwH to understand the decisions they make around gene therapy, and to prepare them mentally and physically for the infusion and what lies beyond. It is important to emphasise that it is not just specialist or research colleagues who need to know about gene therapy. PwH may canvass opinion from staff at all levels within the haemophilia centre, or from link nurses they come into contact with in other medical wards. We therefore believe that all members of the multidisciplinary team and all staff within a haemophilia treatment centre should be armed with the knowledge and confidence to answer questions about gene therapy. At present, health care professionals have clear gaps in knowledge related to gene therapy [24,32,33]. Education programs should target understanding of the fundamentals of gene therapy in general, and specifically how gene therapy is being developed as a treatment approach for haemophilia A and B [24].
Successful gene therapy will likely reduce the level of care that people with haemophilia need [34]. At present, and influenced by the Covid-19 pandemic, much of haemophilia care is delivered outside the centre, with the nurse playing a key role in supporting people remotely by phone or email, or through liaison with local services [28,35]. We might expect this same pattern to hold for people undergoing gene therapy after the initial monitoring period. However, not all people with haemophilia will be eligible for gene therapy [1,34], and it will be important to manage expectations – from very early in the discussions, and throughout the pre-infusion journey [36].
Nurses will have an important role to play in mental support and counselling for those who desire gene therapy but are ineligible, as well as for those who embark on the treatment and may struggle to come to terms with the change, or who find the initial follow-up period difficult. Although not couched as a concern, when discussing their concerns and questions around infusion day, the responses of two participants raised the possibility of a need for greater counselling in advance of having gene therapy to help PwH deal with its potentially life-changing nature. Before gene therapy, many PwH plan their lives around their disease, but afterwards they have to learn to adapt to live without it. When analysing the responses relating to the impact of gene therapy on daily life, the three participants with more recent infusions (up to eight months previously) expressed greater degrees of caution in their response than those who were 10 or more months out from their gene therapy infusion. This may be indicative of a wariness early on and during the follow-up periods, giving way to a growing sense of confidence as more time passes.
Our respondents also indicated that the frequency of follow-up appointments is inconvenient. While we acknowledge that all were participating in clinical trials of investigational gene therapy products, which inherently require much more follow-up and monitoring than may be required in normal clinical practice, nurses may be well-positioned to help provide support during the follow-up period. It has already been demonstrated that a key barrier to adherence to current prophylaxis is a lack of understanding about the underlying disease [31]. Again, education is key to driving optimal outcomes. Ensuring long-term follow-up and adherence to the monitoring regimen for gene therapy will therefore become an important part of the nurse workload as people with haemophilia transition to new care patterns post infusion.
Limitations
We acknowledge that there are some limitations to our study. First, it is possible that the interview format meant that respondents did not feel able to offer negative insights, which could have biased our findings. Respondents may have been more candid with a third-party interviewer, or if responses had been collected in an anonymised survey. Additionally, our interviews took place in a small sample, and may not be representative of the wider haemophilia population. It is important to recognise that the positive views canvassed in this study from those who have made the decision to receive an investigational gene therapy product contrast with perspectives collected by other groups. Qualitative interviews conducted in the Netherlands with people with haemophilia and their families to collect views on novel therapies found that there are concerns about the short- and long-term safety of new treatments, and many believe the effects of gene therapy are not yet fully understood [21]. A larger study would be required to make any generalisable findings about experiences on the gene therapy journey.
CONCLUSION
As with any new therapy, there is a need to make shared decisions to proceed with gene therapy [23]. All health care professionals working with people considering gene therapy should make it clear that research is ongoing, and that there are remaining evidence gaps [36]. Although the participants in this study had generally positive perspectives with regard to their experience of receiving gene therapy, there remains a need for education and support. As more people with haemophilia undertake gene therapy, it will be important to continue to analyse their experiences to help support them at all steps in the journey. Nurses will play a pivotal role in the delivery of gene therapy for haemophilia; they are a key and trusted source of information for PwH and are well positioned to offer general counselling and support. All health care professionals within the haemophilia treatment centre should be armed with the knowledge and confidence to answer questions about gene therapy from PwH and their families.