Bleeding severity in haemophilia correlates with the level of clotting factor; those with the lowest clotting factor levels (<0.01 IU/dL) suffer significant spontaneous bleeding into the large synovial joints (knees, elbows and ankles) of the musculoskeletal system. Over time, joint bleeds result in irreversible joint damage and limitations in activities of daily living.
Prophylactic administration of clotting factor prevents the development of joint damage [1,2] and is now accepted as the standard of care for children born with severe haemophilia [3]. As a result, boys born with severe haemophilia in the UK are now routinely treated with prophylaxis [4] and are increasingly growing up to be active members of society with near-normal lifestyles and lifespans. In the UK, there are about 900 boys aged ≤18 years with severe haemophilia [5].
Prophylaxis demands intravenous injections of clotting factors, which are given at home by boys or their family members, every two to three days. This results in a reduced bleed frequency, with bleeds usually only occurring following significant trauma or sporting injury. Treatment costs in the region of £50,000-100,000 per patient per year.
Haemophilia centres in the UK now routinely use the Haemophilia Joint Health Score (HJHS) performed by haemophilia physiotherapists to assess joint health in clinical practice. Assessments are performed at routine follow up clinics provided no bleeds have occurred in the preceding four weeks. This is a well-validated tool that accurately reflects early joint changes and can be used to monitor joint health, damage and improvement. [6,7,8]
However, as pressure grows on health care budgets, commissioners of health care are increasingly requiring haemophilia treaters to justify the use of high dose and/or intensive prophylaxis using additional measures of functional outcome and quality of life (Table 1). These so-called patient-reported outcome measures assess the quality of care delivered to patients from the patient perspective [9] by questionnaire completion by boys with haemophilia. However, even when collected these data are often not analysed and used.
Table 1
Patient reported outcome measures used in haemophilia research and care
| Measure | Data collection method |
|---|---|
| PedHAL | A score developed to measure the impact of haemophilia on self-perceived functional abilities in children [10,11], based on the adult HAL questionnaire. It consists of 53 items in seven domains: |
| • Sitting/kneeling/standing (10 items) | |
| • Functions of the legs (11 items) | |
| • Functions of the arms (6 items) | |
| • Use of transportation (3 items) | |
| • Self-care (9 items) | |
| • Household tasks (3 items) | |
| • Leisure activities and sports (11 items) | |
|
|
|
| HEP-Test-Q | A short questionnaire developed for the assessment of subjective physical performance in adults with haemophilia [12] and is being validated in children. It consists of 25 items pertaining to four subscales 'mobility', 'strength & coordination', 'endurance' and 'body perception'. |
|
|
|
| Haemo-QOL | A haemophilia-specific self-report questionnaire for the assessment of health-related quality of life, available in three age-group versions for children aged 4-7 years (version I, which contains 21 items), 8-12 years (version II which contains 64 items) and 13-16 years (version III, which contains 77 items). Each version consists of 9–11 subscales, depending on the age group [13]. The short version for children aged 8-16 years (consisting of 35 questions) is being used in this study [14]. |
It is unclear whether these scores add to the data already routinely collected in haemophilia centres and whether these measures are acceptable to boys with haemophilia. Many of the survey questionnaires that boys with haemophilia are asked to complete are long and unengaging, and completion rates are low. Furthermore, questionnaires that focus on the assessment of disability (such as PedHAL) may not be fully appropriate for children and teenagers who have benefited from prophylaxis and who do not consider themselves “disabled”.
Commissioners demand that these questionnaires are completed, but not that they are analysed or that the results are discussed with individual boys to improve their health. Analysis of the paper questionnaires involves data entry into a database, where data inputting errors may occur, and scoring of the data to result in a series of scores, which may reflect poor functional ability or QoL. The ability to complete the questionnaires on-line would result in real-time results that could be discussed with boys at routine clinic appointments. This may result in additional benefits, such as being compared to other boys treated in individual centres, those in a certain age group across a region or nation, and may enhance treatment concordance.
Materials and methods
Study Design and aims
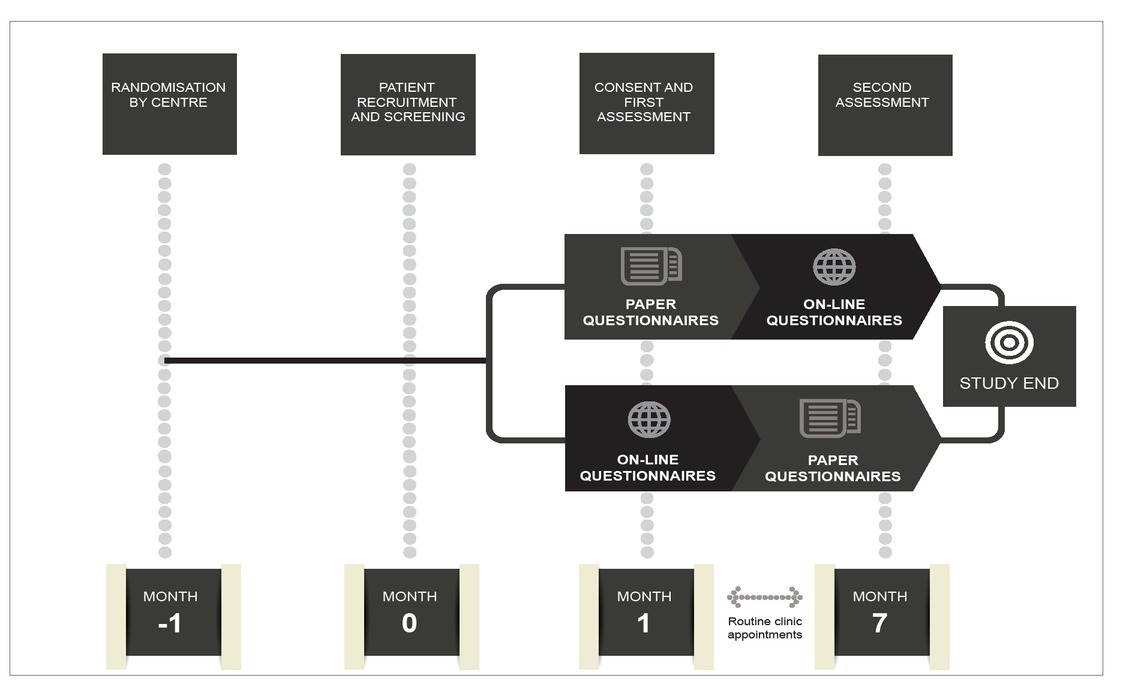
The Study Of physical Function In adolescenTs with haemophilia (SO-FIT) is a multicenter, randomized crossover study. The study design is summarised in Figure 1.
The principal study objective is to determine whether self-perceived functional ability and quality of life (QoL) correlate with joint scores as measured by the Haemophilia Joint Health Score (HJHS) (version 2.1) in children and young people with severe haemophilia.
Secondary aims are to determine:
Whether measures of functional outcome (PedHAL and HEP-Test-Q) correlate with accepted quality of life measures (Haemo-QoL short version).
Whether the HEP-Test-Q is as good a measure of functional outcome as the PedHAL and acceptable to boys with haemophilia.
Whether measures of functional outcome (PedHAL and HEP-Test-Q) and quality of life (Haemo-QoL short version) are acceptable to boys with haemophilia.
Whether measures of functional outcome (PedHAL and HEP-Test-Q) and quality of life (Haemo-QoL short version) are completed more fully and frequently if made available on mobile devices rather than paper
Study conduct
The SO-FIT study is being conducted in 15 UK haemophilia centres where nurse/physiotherapist dyads work together: the nurse will supply participant questionnaires and participant demographic data, the physiotherapist will perform the HJHS and provide raw score data. The study is being coordinated by Haemnet, and is being funded under a medical education grant provided by Pfizer.
Study subjects
SO-FIT will recruit approximately 120 boys in order to ensure that 100 boys complete both the screening and follow up assessments: in order to avoid centre bias a maximum of 20 boys will be allowed per centre. All eligible boys will be identified by the participating centre staff using a consecutive approach in order to limit selection bias. Boys are eligible for inclusion if they have severe haemophilia A or B with or without inhibitors and are aged 8-16 years at first visit. In addition, boys must be able to speak/write English, and able to assent to participation in the study. They must be able to self-complete the questionnaires, and be expected to remain at the same centre for the study duration.
One month before the boys are due to be reviewed at the haemophilia centre, information sheets will be posted to the parents of those identified as potential study participants. Parents who agree their child may participate in the study will be asked to give study information to their child in order to allow sufficient time for the parents and boys to consider study participation. Consent to join the study will be requested by the haemophilia nurse specialist on the day of the clinic visit.
Data collection
Basic patient data will be collected by the study nurse at each centre, as summarized in Table 2. The HJHS will be performed by the centre physiotherapist, and joint score sheets will be completed along with the demographic data.
Table 2
Summary of patient data to be collected by the study nurse at each participating centre in the SO-FIT study
In addition, the study uses data collected from routine clinical assessment (the HJHS) as well as questionnaires (PedHAL, HEP-Test-Q and Haemo-QoL short version) that have been validated by use in boys with haemophilia across a variety of ages. Those who agree to take part in the study will be asked to come to the consultation 40 minutes before a routine appointment in order to complete the questionnaires, whether on paper or on a tablet computer.
Centres will be randomized to use either paper or mobile device technology for the first assessment and will then cross-over to the alternate method for the second assessment (Figure 1). Thus, during the study, each participant will be assessed twice. In each case, the second assessment will be followed by an additional questionnaire seeking information on boys’ preferences for paper or online surveys together with feedback about their participation in the study.
Data analysis
All study data will be entered into a password-protected database and will be analysed centrally by the haemophilia research coordinator at Great Ormond Street Hospital for Children NHS Foundation Trust. The study population will be described using standard descriptive statistics for clinical and demographic characteristics. Study questionnaires will be analysed using descriptive statistical techniques listing the usual values such as incidence, mean, median, standard deviation, minimum, maximum (quantitative variables) using scoring, recoding and normalization of score methodology for each questionnaire. HJHS data will be entered for each child’s joints and correlation of self and physiotherapist assessment between HJHS, PedHAL and HEP-Test-Q will be performed. The impact of functional impairment will be modeled against reported quality of life to establish its impact on quality of life.
Data security and confidentiality
All data will be safeguarded according to the Data Protection Act. Access to the data will be via password-protected systems. While individual haemophilia centres will know study participants by name, all data about participants will be anonymised to study number. No children will be named and confidentiality will be maintained.
Discussion
In its 2010 White Paper, Equity and Excellence: Liberating the NHS [15], the government espoused a vision in which patients and clinicians reach shared decisions: ‘Shared decision-making will become the norm: no decision about me without me.’ Yet, as noted by the Patient-Centered Outcomes Research Institute, if patients are to participate in decision-making and research then the outcomes used to assess and compare treatments should be meaningful to patients and reflect what they actually want to achieve from treatment [16]. Although the use of patient reported outcome measures is becoming increasingly common in many fields of research, the patient voice is rarely heard in respect of the outcome measures used in haemophilia, particularly if the voice is that of a child/young person.
Compared to people living with other long-term conditions, there is very little data available on the day-today effect of haemophilia on young people’s lives. Much of the available published data focuses on the impact of blood-borne viral infections on the haemophilia community, an issue that due to modern treatment approaches is no longer relevant for the cohort of boys in this study. This study will, for the first time, ensure that the views of boys with haemophilia are heard with respect to treatment outcome measures and the relevance that these have on their daily lives.
Boys with haemophilia are already required to complete treatment diaries, yet collecting these data accurately is a significant challenge to healthcare professionals.
In addition, healthcare commissioners frequently request collection of patient-level data on measures of physical function and quality of life. In a recent focus group with 12 boys aged 12-17 years, we learned that boys would be more willing to complete data about themselves if the relevance of the data collection was clear to the individual. Furthermore, data collection would be enhanced if data entry was simple and offered feedback at an individual level, so that each young person could see how ‘well’ he was doing in comparison with his peers with haemophilia.
Clearly, patients have a right to their own data. Therefore, it is our intention to publish the anonymised results of this study on the SixVibe patient website (www.sixvibe.com). Furthermore, through that website, each study participant will be given access to his own results so that he can compare these with anonymised data from other boys within his age group, or those treated at his haemophilia centre or from the area of the country in which he lives.