A survey of specialist haemophilia nurses in Europe and Canada indicates a need for education to promote confidence and competence to support effective treatment outcomes for people with haemophilia B.

Some clinicians believe that haemophilia B is associated with less bleeding than haemophilia A [1]. In one comparison of age-matched people with severe haemophilia A or B, haemarthrosis was more common with worse joint scores in haemophilia A [2]. However, such differences have not always been clearly demonstrated. Although milder arthropathy is reported in haemophilia B [3], there appears to be little difference in health-related outcomes between the disorders. One survey of people with severe haemophilia A or B between 1998 and 2013, when treatment largely comprised on-demand factor replacement, found no significant difference in major bleeding events or resulting admissions [4].
This conflicting evidence fosters a belief that haemophilia A is intrinsically more severe than haemophilia B. This could lead health professionals to manage the two disorders differently but should not be interpreted as meaning a person with haemophilia B cannot have bleeds or that complications are not as severe as experienced in haemophilia A [5]. Further, early use of prophylaxis has created a haemophilia population with different risks from older populations. The RODIN study found no differences in severity and variation in bleeding phenotype in children with haemophilia [6], most of whom (73.5% haemophilia A and 85.9% haemophilia B) received prophylaxis, on average started within a year of diagnosis. As current clinical practice reduces bleed rates [7], differences between haemophilia A and B are becoming difficult to measure unless based on historical age cohorts and access to treatment.
From a clinical perspective, the important question is not whether haemophilia A affects individuals more than haemophilia B, but the severity of bleeding and how it is managed to minimise complications. We surveyed specialist haemophilia nurses to discern their opinions about the impact of haemophilia B compared with haemophilia A.
METHODS
Haemnet Horizons (https://www.haemnet.com/resources/horizons) is an international working group of haemophilia nurses convened by Haemnet to foster research and develop clinical practice. Following a Haemnet Horizons discussion, an online survey comprising 25 questions about perceptions of management and treatment of haemophilia B was devised (see Appendix). Haemnet Horizons members invited nurse colleagues in their home countries to complete the survey between July and September 2020. As an anonymous voluntary survey of health care providers, ethical approval was unnecessary.
Data analysis
Descriptive data are presented, with medians and ranges where appropriate.
RESULTS
Respondents
Fifty-nine nurses completed the survey. Most were from Europe (Denmark 5, Netherlands 15, Spain 10, Sweden 3, UK 13); 13 were Canadian. Seventeen treated adults (29%), 14 treated children (24%), and 28 treated both (47%). Five had worked in haemophilia <2 years (8.5%), 17 for 2–5 years (29%), 13 for 6–10 years (22%), 16 for 10–20 years (27%), and 7 for >20 years (12%).
Differences between haemophilia A and B
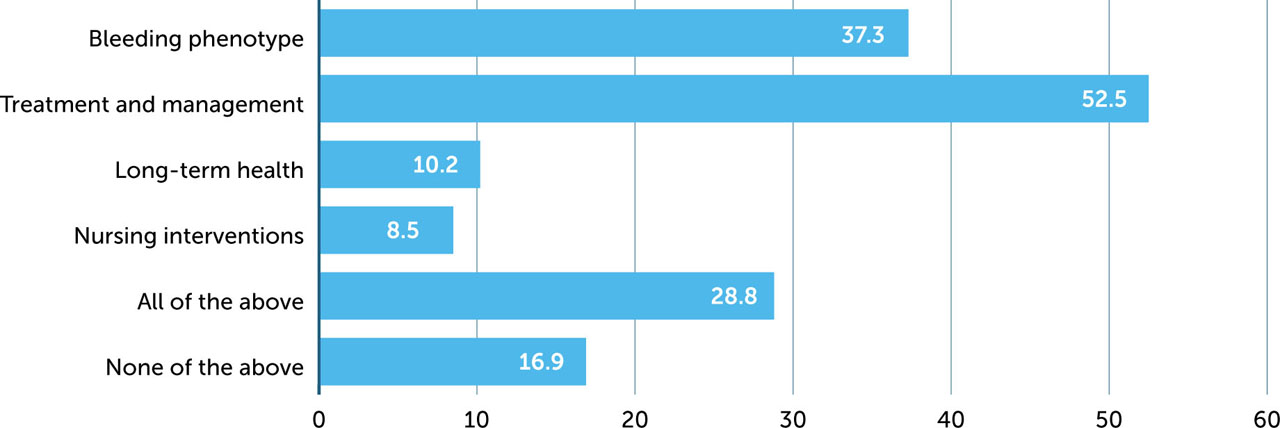
Over one third of respondents stated that the bleeding phenotypes in haemophilia A and B are different, and half that treatment and management are different (Figure 1). This may reflect different therapeutic options (e.g. non-factor treatment for mild haemophilia A, access to extended half-life (EHL) products, infusion frequency). By contrast, less than half believe there are differences in long-term health or nursing interventions. Approximately one in six respondents stated there are no differences; however, 29% stated that management of the two disorders is different in all these respects.
Figure 1
Areas of perceived difference between haemophilia A and B
Provision of care
Most respondents stated there was no difference in the frequency with which patients were seen in clinic (85% for those with severe haemophilia, 76% for moderate, and 73% for mild). A minority stated that patients with haemophilia B were seen less often (6.8% for those with a severe phenotype, 19% for moderate, and 27% for mild).
Nearly all respondents (92%) stated that patients with severe haemophilia B routinely received prophylaxis. The proportion was far lower for patients with a moderate (24%) or mild (5.1%) phenotype. Similarly, most stated that patients with severe or moderate haemophilia received regular joint assessment (81% and 68% respectively), whereas only 47% reported them being offered to those with mild haemophilia. Only 58% of respondents stated that women with haemophilia B were seen as often as their male counterparts. Fifty-eight per cent of nurses had treated a person with haemophilia B and an inhibitor.
Experience with factor IX products
Most respondents had experience of using standard half-life (SHL) factor IX (FIX) products. Of 36 respondents reporting experience with the most frequently used EHL FIX (eftrenonacog alfa; Alprolix®, Swedish Orphan Biovitrum AB/Sanofi [8]), 19 reported experience with at least one other EHL FIX (including Albutrepenonacog alfa; Idelvion®, CSL Behring [9] and Nonacog beta pegol; Refixia/Rebinyn®, Novo Nordisk [10]). Of 23 respondents who did not report experience with eftrenonacog alfa, six reported experience with at least one of the other two EHL FIX products and 17 reported no experience with the products listed (Table 1).
Table 1
Experience of use of factor IX products
|
PRODUCT
|
N
|
%
|
|
Standard half-life |
|
Nonacog alfa |
Benefix [31]
|
47 |
80% |
|
Nonacog gamma |
Rixubis [32]
|
11 |
19% |
|
Extended half-life |
|
Albutrepenonacog alfa |
Idelvion [9]
|
22 |
37% |
|
Eftrenonacog alfa |
Alprolix [8]
|
36 |
61% |
|
Nonacog beta pegol |
Refixia/Rebinyn [10]
|
13 |
22% |
|
Other |
|
17 |
29% |
Forty-eight respondents identified who is involved in the decision to initiate treatment with EHL products. Of these, 19% stated it was a clinical decision made by a health care professional (HCP); the remainder said it was a decision made jointly by the patient and HCP (59%) or purely by the patient (3%).
Pharmacokinetic (PK) assessment
Fifty-nine percent of respondents stated that patients receiving regular treatment with FIX undergo PK assessment; a further 37% stated this occurred only when switching between products. Routine measurement of trough FIX activity at each clinic visit, as a surrogate marker for PK assessment, was reported by 58%.
Nine respondents did not positively endorse trough FIX activity as a marker of treatment efficacy. The remainder stated unequivocally that it is relevant, or relevant when interpreted in the context of clinical outcomes such as bleeding events and joint assessment.
Thirty-five respondents (59%) stated they were aware that FIX undergoes extravascular distribution [11,12,13]. Thirty-three commented on the clinical significance of this, of whom 26 (44% of all respondents) correctly alluded to or stated that extravascular FIX contributes to haemostasis but is not measured by routine blood tests [11].
Using EHL FIX products
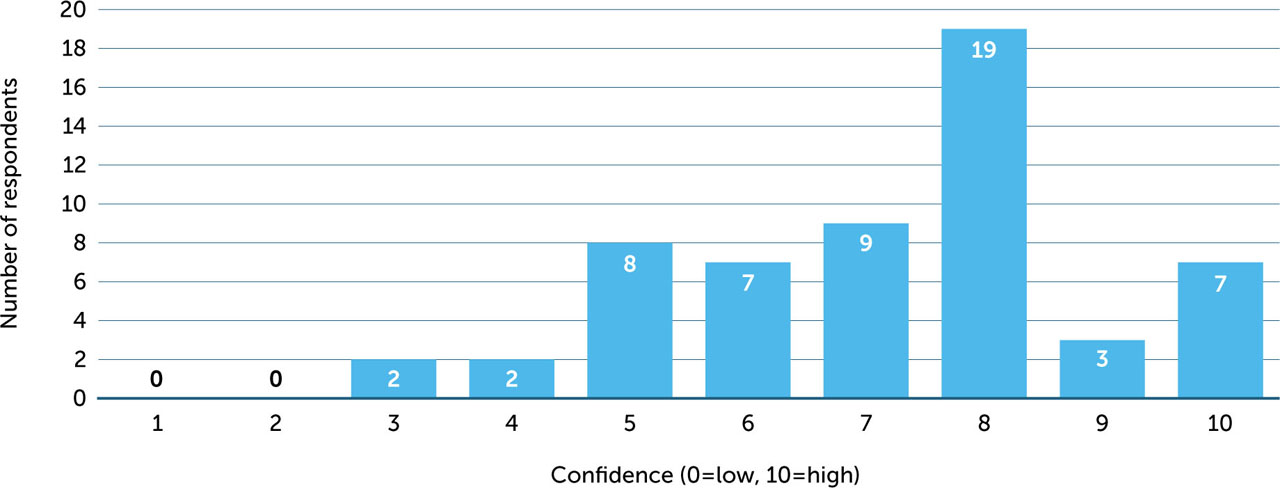
Respondents were asked to rate their confidence in using EHL FIX products on a scale of 1 (low) to 10 (high). The mean score was 7.1 (median 8, range 3–10) (Figure 2). There was no apparent association between level of confidence and responses to other questions about management. Of 12 nurses who rated their confidence at ≤5, three had worked in haemophilia care for 2–5 years, three for 6–10 years, and six for 11–20 years.
Figure 2
Respondents' self-reported rating of confidence using EHL FIX products (1 = not very confident; 10 = very confident)
Twenty-three respondents (39%) reported using EHL products in the management of acute bleeding episodes in patients using on-demand therapy, and 27 (46%) used them in the management of surgery or dental procedures. Almost all (93%) used the patient's current product to cover surgery or dental procedures; two respondents reported switching to SHL products. The decision to use EHL products in this context was made by the nurse (14%), the centre director (24%), the consultant (36%), and the multidisciplinary team (MDT) (49%) – categories were not mutually exclusive.
Fifty-six respondents (95%) provided information about acute bleed management. Nineteen (34%) reported advising the patient to contact the treatment centre first. Nine (15%) advised patients to self-treat as a first step, then to contact the treatment centre for advice either routinely, if the bleeding did not stop, or if they needed further advice. Seven (12%) stated their response would depend on the severity of bleeding; three (5.1%) mentioned the use of a treatment plan; nine (15%) stated they advised patients to use their usual factor. Two respondents stated they did not provide advice.
Perceived satisfaction with treatment and future treatment for haemophilia B
Forty-two respondents (71%) stated that the needs of patients with haemophilia B and their families are addressed in the same way as for those with haemophilia A. Of those who did not, several noted that haemophilia A receives more resources or attention, there has been less research on haemophilia B, and less access to EHL-FIX products.
About half of respondents (47%) felt that EHL-FIX is likely to offer the optimal treatment for haemophilia B in 2025; 29% thought this would be gene therapy and 19% thought it would be a novel agent such as fitusiran [14] or concizumab [15]. Two thought an SHL product would remain the optimal treatment.
When asked to identify unmet needs for people with haemophilia B, respondents suggested a diverse range of issues, including improved information about haemophilia B and its treatment; management of bleeds; access to physiotherapy and psychosocial care; patient support and access to peers; attention to age-related morbidity; more research and new treatments, including an alternative to intravenous replacement therapy. Eighteen respondents identified specific topics they would like more information about, including use of EHL products, inhibitors, gene therapy, new products, PK and extravascular distribution of FIX, patient education and management.
DISCUSSION
This survey provides a snapshot of how specialist haemophilia nurses perceive haemophilia B. The respondents collectively had long experience of haemophilia care; their views reflect the well-resourced care available in specialist centres in northern Europe and Canada.
A significant minority (29%) believed that haemophilia B is managed differently from haemophilia A, perhaps because of access to different therapeutic options, including the longer time interval between infusions of both SHL and EHL-FIX to manage bleeding. Conversely, as nurses see more bleeds in people with haemophilia A than in people with haemophilia B, they may put undue weight on this and wrongly believe that familiar events are more significant than those seen less often [16,17].
The majority of nurses who completed the survey believed that outcomes and nursing interventions are not different, with most reporting similar frequencies of clinic attendance for all with haemophilia. Most people with severe haemophilia B were treated with prophylaxis in line with the latest World Federation of Hemophilia guidance [18]; whereas relatively few with moderate or mild haemophilia B were, including women. Interestingly, while the overall inhibitor rate in haemophilia B is reported as 6% [19], 58% of nurses had treated a person with haemophilia B and an inhibitor, reflecting the complex nature of care required and delivered by specialist haemophilia nurses.
It is surprising that 20% of respondents reported no experience of the use of nonacog alfa (BeneFix), a frequently used standard half-life factor IX product introduced in Europe and North America in the late 1990s [20]. Nine of these respondents were from Canada, three from Spain and two from Scandinavia; other respondents in each of these regions reported experience with this product. Despite being relatively new to the haemophilia B treatment armamentarium [21] only 29% of respondents reported no experience with one of the three EHL FIX products. These findings raise the possibility of an unmet educational need and large differences in clinical practice between centres.
It is evident that many nurses have direct experience of treatment with EHL products; however, few have autonomy in choosing treatment. Prescribing is an extended clinical role undertaken by competent nurses in only a few countries [22]. There are also national prescribing protocols based on purchasing tenders which pre-select product availability [23,24]. Most respondents reported joint decision making by the patient and clinician around using an EHL-FIX, but about one fifth stated this was a purely clinical decision. Shared decision making between patients and clinicians is increasingly important in haemophilia care [25] but may challenge patients and practitioners [26]. The level of confidence in using EHL products was generally high, though 12 respondents (20%) rated their confidence at 50% or lower with limited understanding of the extravascular distribution of FIX, and how this affects PK and dose calculation and requires further education to support patient knowledge. Many of these nurses had long experience of haemophilia care, suggesting a need for improved current awareness.
The survey revealed variation in the advice and support given to patients experiencing acute bleeds. A treatment plan that includes a protocol for self-treating is now commonplace, but 34% of respondents said their advice to the patient was first to contact the treatment centre and a further 15% advised this after initial self-treatment. The survey did not provide information on whether this was unique to patients using EHL products or if it was a general rule.
About one-third of respondents felt patients with haemophilia B and their families receive less attention – in terms of access to new products, research effort, resources – than those with haemophilia A. This contrasts with the extensive B-HERO-S studies, which report that people with haemophilia B suffer pain, anxiety and depression [27], issues with relationships [28] and sexual health [29], and impaired quality of life [30]. They also identified a variety of unmet needs for themselves (largely about information and the role of the extra-vascular space in PK in EHL-FIX) and their patients (largely access to or need for improved care), though these were spontaneous rather than systematic evaluations.
Limitations
This survey reflects the views of a relatively small and self-selected group of specialist nurses working in well-developed health services. Possible differences in the management of haemophilia A and B were identified by respondents' perceptions, not a direct comparison of patients.
CONCLUSIONS
Although not consistent with the experiences of the majority in our survey, some specialist haemophilia nurses believe that haemophilia A and B are managed differently beyond factor dosing schedules. While many have direct experience of using EHL-FIX products, and many believe these will remain the optimal treatment option for haemophilia B over the next five years, levels of confidence vary. There is also variation between haemophilia treatment centres in the advice given to patients around managing acute bleeds and decision-making around treatment choices. There is a need for education to promote confidence and competence to further support effective treatment outcomes for haemophilia B.
ACKNOWLEDGEMENTS
We thank all the nurses who completed the survey.
The Haemnet Horizons initiative was funded by an unrestricted educational grant from Sobi AB.
This paper reports on a survey of health care professionals and did not require research board approval.
REFERENCES
Castaman G, Matino D. Hemophilia A and B: molecular and clinical similarities and differences. Haematologica 2019; 104: 1702–1709. doi: 10.3324/haematol.2019.221093.
G CastamanD MatinoHemophilia A and B: molecular and clinical similarities and differencesHaematologica20191041702170910.3324/haematol.2019.221093
Melchiorre D, Linari S, Manetti M, et al. Clinical, instrumental, serological and histological findings suggest that hemophilia B may be less severe than hemophilia A. Haematologica 2016; 101: 219–25. doi: 10.3324/haematol.2015.133462.
D MelchiorreS LinariM ManettiClinical, instrumental, serological and histological findings suggest that hemophilia B may be less severe than hemophilia AHaematologica20161012192510.3324/haematol.2015.133462
Kihlberg K, Baghaei F, Bruzelius M, et al. Treatment outcomes in persons with severe haemophilia B in the Nordic region: The B-NORD study. Haemophilia 2021; 27(3): 366–374. doi: 10.1111/hae.14299.
K KihlbergF BaghaeiM BruzeliusTreatment outcomes in persons with severe haemophilia B in the Nordic region: The B-NORD studyHaemophilia202127336637410.1111/hae.14299
Shih MY, Wang JD, Yin JD, et al. Differences in major bleeding events between patients with severe hemophilia A and hemophilia B: a nationwide, population-based cohort study. Clin Appl Thromb Hemost 2019; 25: 1076029619888023. doi: 10.1177/1076029619888023.
MY ShihJD WangJD YinDifferences in major bleeding events between patients with severe hemophilia A and hemophilia B: a nationwide, population-based cohort studyClin Appl Thromb Hemost201925107602961988802310.1177/1076029619888023
Dolan G, Benson G, Duffy A, et al. Haemophilia B: Where are we now and what does the future hold? Blood Rev 2018; 32(1): 52–60. doi: 10.1016/j.blre.2017.08.007.
G DolanG BensonA DuffyHaemophilia B: Where are we now and what does the future hold?Blood Rev2018321526010.1016/j.blre.2017.08.007
Clausen N, Petrini P, Claeyssens-Donadel S et al; PedNet and Research of Determinants of Inhibitor development (RODIN) Study Group. Similar bleeding phenotype in young children with haemophilia A or B: a cohort study. Haemophilia 2014; 20: 747–55. doi: 10.1111/hae.12470.
N ClausenP PetriniS Claeyssens-DonadelPedNet and Research of Determinants of Inhibitor development (RODIN) Study GroupSimilar bleeding phenotype in young children with haemophilia A or B: a cohort studyHaemophilia2014207475510.1111/hae.12470
Osooli M, Steen Carlsson K, Astermark J, et al. Surgery and survival in birth cohorts with severe haemophilia and differences in access to replacement therapy: The Malmö experience. Haemophilia 2017; 23(5): e403–e408. doi: 10.1111/hae.13302.
M OsooliK Steen CarlssonJ AstermarkSurgery and survival in birth cohorts with severe haemophilia and differences in access to replacement therapy: The Malmö experienceHaemophilia2017235e403e40810.1111/hae.13302
Iorio A, Fischer K, Blanchette V, Rangarajan S, Young G, Morfini M; Pharmacokinetic (PK) Expert Working Group of the International Prophylaxis Study Group (the IPSG). Tailoring treatment of haemophilia B: accounting for the distribution and clearance of standard and extended half-life FIX concentrates. Thromb Haemost 2017; 117(6): 1023–1030. doi: 10.1160/TH16-12-0942.
A IorioK FischerV BlanchetteS RangarajanG YoungM MorfiniPharmacokinetic (PK) Expert Working Group of the International Prophylaxis Study Group (the IPSG)Tailoring treatment of haemophilia B: accounting for the distribution and clearance of standard and extended half-life FIX concentratesThromb Haemost201711761023103010.1160/TH16-12-0942
Negrier C, Knobe K, Tiede A, et al. Enhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia B. Blood 2011; 118(10): 2695–701. doi: 10.1182/blood-2011-02-335596.
C NegrierK KnobeA TiedeEnhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia BBlood201111810269570110.1182/blood-2011-02-335596
Santagostino E, Negrier C, Klamroth R, et al. Safety and pharmacokinetics of a novel recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in hemophilia B patients. Blood 2012; 120(12): 2405–11. doi: 10.1182/blood-2012-05-429688.
E SantagostinoC NegrierR KlamrothSafety and pharmacokinetics of a novel recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in hemophilia B patientsBlood20121201224051110.1182/blood-2012-05-429688
Machin N, Ragni MV. An investigational RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and B. J Blood Med 2018; 9: 135–140. doi: 10.2147/JBM.S159297.
N MachinMV RagniAn investigational RNAi therapeutic targeting antithrombin for the treatment of hemophilia A and BJ Blood Med2018913514010.2147/JBM.S159297
Shapiro AD. Concizumab: a novel anti-TFPI therapeutic for hemophilia. Blood Adv 2021; 5(1): 279. doi: 10.1182/bloodadvances.2019001140.
AD ShapiroConcizumab: a novel anti-TFPI therapeutic for hemophiliaBlood Adv20215127910.1182/bloodadvances.2019001140
Kahneman D, Tversky A. Judgement under uncertainty: heuristics and biases. Science 1974; 185: 1124–1131. doi: 10.1126/science.185.4157.1124.
D KahnemanA TverskyJudgement under uncertainty: heuristics and biasesScience19741851124113110.1126/science.185.4157.1124
Tversky A, Kahneman D. Availability: A heuristic for judging frequency and probability. Cognitive Psychology 1973; 5: 207–232. doi: 10.1016/0010-0285(73)90033-9.
A TverskyD KahnemanAvailability: A heuristic for judging frequency and probabilityCognitive Psychology1973520723210.1016/0010-0285(73)90033-9
Srivastava A, Santagostino E, Dougall A, et al.; WFH Guidelines for the Management of Hemophilia panelists and co-authors. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020; 26 Suppl 6: 1–158. doi: 10.1111/hae.14046.
A SrivastavaE SantagostinoA DougallWFH Guidelines for the Management of Hemophilia panelists and co-authorsWFH Guidelines for the Management of Hemophilia, 3rd editionHaemophilia202026Suppl 6115810.1111/hae.14046
Mansouritorghabeh H, Mohades ST. Is the detection of factor IX inhibitors in hemophilia B orphan than factor VIII inhibitors in hemophilia A? A concise, systematic review. Cardiovasc Hematol Disord Drug Targets 2020; 20(3): 185–190. doi: 10.2174/1871529X20666200701104143.
H MansouritorghabehST MohadesIs the detection of factor IX inhibitors in hemophilia B orphan than factor VIII inhibitors in hemophilia A? A concise, systematic reviewCardiovasc Hematol Disord Drug Targets202020318519010.2174/1871529X20666200701104143
Rendo P, Smith L, Lee HY, et al. Nonacog alfa: an analysis of safety data from six prospective clinical studies in different patient populations with haemophilia B treated with different therapeutic modalities. Blood Coagul Fibrinolysis 2015; 26(8): 912–8. doi: 10.1097/MBC.0000000000000359.
P RendoL SmithHY LeeNonacog alfa: an analysis of safety data from six prospective clinical studies in different patient populations with haemophilia B treated with different therapeutic modalitiesBlood Coagul Fibrinolysis2015268912810.1097/MBC.0000000000000359
Mahlangu JN. Updates in clinical trial data of extended half-life recombinant factor IX products for the treatment of haemophilia B. Ther Adv Hematol 2018; 9(11): 335–346. doi: 10.1177/2040620718802606.
JN MahlanguUpdates in clinical trial data of extended half-life recombinant factor IX products for the treatment of haemophilia BTher Adv Hematol201891133534610.1177/2040620718802606
Pollard D, Harrison C, Dodgson S, et al. The UK haemophilia specialist nurse: Competencies fit for practice in the 21st century. Haemophilia 2020; 26(4): 622–630. doi: 10.1111/hae.14002.
D PollardC HarrisonS DodgsonThe UK haemophilia specialist nurse: Competencies fit for practice in the 21st centuryHaemophilia202026462263010.1111/hae.14002
O'Mahony B, Noone D, Prihodova L. Survey of coagulation factor concentrates tender and procurement procedures in 38 European Countries. Haemophilia 2015; 21(4): 436–43. doi: 10.1111/hae.12720.
B O'MahonyD NooneL PrihodovaSurvey of coagulation factor concentrates tender and procurement procedures in 38 European CountriesHaemophilia20152144364310.1111/hae.12720
Hay CR. Purchasing factor concentrates in the 21st century through competitive tendering. Haemophilia 2013; 19(5): 660–7. doi: 10.1111/hae.12169.
CR HayPurchasing factor concentrates in the 21st century through competitive tenderingHaemophilia2013195660710.1111/hae.12169
Garner K, Guelcher C, Pollard D. The use of rIX-FP in patients with haemophilia B: A nurse's perspective. J Haem Pract 2021; 8(1): 86–97. doi: 10.17225/jhp00180.
K GarnerC GuelcherD PollardThe use of rIX-FP in patients with haemophilia B: A nurse's perspectiveJ Haem Pract202181869710.17225/jhp00180
Valentino LA, Blanchette V, Negrier C, et al. Personalising haemophilia management with shared decision making. J Haem Pract 2021; 8(1): 69–79. doi: 10.17225/jhp00178.
LA ValentinoV BlanchetteC NegrierPersonalising haemophilia management with shared decision makingJ Haem Pract202181697910.17225/jhp00178
Buckner TW, Sidonio R Jr, Witkop M, et al. Correlations between patient-reported outcomes and self-reported characteristics in adults with hemophilia B and caregivers of children with hemophilia B: analysis of the B-HERO-S study. Patient Relat Outcome Meas 2019; 10: 299–314. doi: 10.2147/PROM.S219166.
TW BucknerR Sidonio JrM WitkopCorrelations between patient-reported outcomes and self-reported characteristics in adults with hemophilia B and caregivers of children with hemophilia B: analysis of the B-HERO-S studyPatient Relat Outcome Meas20191029931410.2147/PROM.S219166
Cutter S, Guelcher C, Hunter S, et al. Mild-severe hemophilia B impacts relationships of US adults and children with hemophilia B and their families: results from the B-HERO-S study. Patient Relat Outcome Meas 2019; 10: 257–266. doi: 10.2147/PROM.S214188.
S CutterC GuelcherS HunterMild-severe hemophilia B impacts relationships of US adults and children with hemophilia B and their families: results from the B-HERO-S studyPatient Relat Outcome Meas20191025726610.2147/PROM.S214188
Blamey G, Buranahirun C, Buzzi A, et al. Hemophilia and sexual health: results from the HERO and B-HERO-S studies. Patient Relat Outcome Meas 2019; 10: 243–255. doi: 10.2147/PROM.S211339.
G BlameyC BuranahirunA BuzziHemophilia and sexual health: results from the HERO and B-HERO-S studiesPatient Relat Outcome Meas20191024325510.2147/PROM.S211339
Buckner TW, Witkop M, Guelcher C, et al. Impact of hemophilia B on quality of life in affected men, women, and caregivers – Assessment of patient-reported outcomes in the B-HERO-S study. Eur J Haematol 2018; 100(6): 592–602. doi: 10.1111/ejh.13055.
TW BucknerM WitkopC GuelcherImpact of hemophilia B on quality of life in affected men, women, and caregivers – Assessment of patient-reported outcomes in the B-HERO-S studyEur J Haematol2018100659260210.1111/ejh.13055
Appendices
APPENDIX
-
In which of the following is there a difference between haemophilia A and B? This question is required.*
Choose as many as you like
In which country do you work? This question is required.*
A Canada
B Denmark
C The Netherlands
D Spain
E Sweden
F United Kingdom
Do you treat
Adults
Children
Both adults and children
For how many years have you worked in haemophilia care?
A Less than 2 years
B 2–5 years
C 6–10 years
D 11–20 years
E More than 20 years
-
Thinking about the severe haemophilia B patients you care for, which of the following statements are correct?
Choose as many as you like
A They are routinely offered prophylaxis with factor IX
B They are seen in clinic as often as haemophilia A patients
C They are seen in clinic less often than haemophilia A patients
D They are offered regular joint assessments with a physiotherapist
E I have never seen a severe haemophilia B patient with an inhibitor
-
Thinking about the moderate haemophilia B patients you care for, which of the following statements are correct?
Choose as many as you like
A They are routinely offered prophylaxis with factor IX
B They are seen in clinic as often as haemophilia A patients
C They are seen in clinic less often than haemophilia A patients
D They are offered regular joint assessments with a physiotherapist
-
Thinking about the mild haemophilia B patients you care for, which of the following statements are correct?
Choose as many as you like
A They are routinely offered prophylaxis with factor IX
B They are seen in clinic as often as haemophilia A patients
C They are seen in clinic less often than haemophilia A patients
D They are offered regular joint assessments with a physiotherapist
-
Thinking about the haemophilia B carriers you care for, if they have low FIX levels, do you see them as often as you see patients with mild haemophilia B?
_______________________________________________
_______________________________________________
_______________________________________________
-
Which of following products do you have experience with?
Choose as many as you like
A Benefix (nonacog alfa)
B Alprolix (eftrenonacog alfa)
C Idelvion (albutrepenonacog alfa)
D Refixia/Rebinyn (nonacog beta pegol)
E Rixubis (nonacog gamma)
F Other FIX products
Did your patients on EHL initiate this treatment choice themselves or was it a clinical decision?
A Own decision
B Clinical decision
C Both
Do your haemophilia B patients receiving regular FIX treatments undergo pharmacokinetic testing?
Do you routinely measure trough levels in haemophilia B patients on prophylaxis at each clinic visit?
-
How relevant do you feel FIX trough levels are as a marker of efficacy for haemophilia B? [Free text]
_______________________________________________
_______________________________________________
_______________________________________________
Are you aware of extravascular distribution of FIX in haemophilia B patients?
-
Please tell us what you understand about the extravascular distribution of FIX in haemophilia B patients. [Free text]
_______________________________________________
_______________________________________________
_______________________________________________
-
What do you advise patients with haemophilia B on extended half-life factor prophylaxis to do if they have a bleeding episode? [Free text]
_______________________________________________
_______________________________________________
_______________________________________________
Do you use an extended half-life product to manage bleeds in ‘on-demand’ haemophilia B patients?
Do you use an extended half-life product to manage surgery or dental work in ‘on-demand’ haemophilia B patients?
For your haemophilia B patients on extended half-life products undergoing surgery or dental work, do you
-
Who makes this decision?
Choose as many as you like
A Nurse
B Centre director
C Consultant
D MDT decision
-
On a scale of 1 (not very confident) to 10 (very confident), how confident are you in managing surgery using extended half-life products?
_______________________________________________
_______________________________________________
_______________________________________________
What do you think is likely to be the optimal treatment for patients with severe haemophilia B in 2025?
A Plasma-derived factor
B Standard half-life recombinant factor
C Extended half-life factor
D Gene therapy
E Novel therapies such as fitusiran or concizumab
-
Do you think the needs of patients/families with haemophilia B are addressed in the same way as those with haemophilia A? [Free text]
_______________________________________________
_______________________________________________
_______________________________________________
-
What, if any, do you think are the unmet needs for people with haemophilia B? [Free text]
_______________________________________________
_______________________________________________
_______________________________________________
-
Is there anything about managing a person/family with haemophilia B that you need more information about? [Free text]
_______________________________________________
_______________________________________________
_______________________________________________


