The development of a patient-reported outcome tool for pain, specific to people with bleeding disorders, could help facilitate the pain assessment and discussion of treatment options in bleeding disorder clinics.

Pain is common among people with bleeding disorders. Approximately 57% of adults with haemophilia over age 18 years report daily joint pain [1], and joint pain is also common among adults with moderate or severe von Willebrand Diseases (VWD), both for those who report joint bleeds (44%) and those who do not (18%) [2]. Pain often interferes with daily activities for those with bleeding disorders and has negative associations with quality of life and mental health [3]. Thirty-nine per cent of adults with haemophilia report that their pain is not well managed, and over half report that they rely on the bleeding disorder care team for pain management advice [4,5]. Guidelines call for increased attention to pain assessment and management in comprehensive care of people with bleeding disorders and highlight the need for condition-specific patient reported outcome (PRO) measures [5,6,7,8,9,10]. To meet that call for action, multidisciplinary bleeding disorder clinicians will need the knowledge, skills, and tools to effectively assess and communicate with patients about pain in order to develop acceptable and impactful treatment plans. Chronic pain is a complex, biopsychosocial experience requiring a comprehensive focused assessment that covers multiple domains, including sensory, affective, and motivational aspects of pain, impact of pain on psychological and physical functioning, and preferred treatment approaches [11,12,13]. No pain assessment tools specific to bleeding disorders were found in the literature that included all of these domains.
Management of pain may require pharmacologic and non-pharmacologic treatment interventions as well as education and skills training for self-management [14–15]. Communication between healthcare providers and patients is a critical first step in developing an assessment-based care plan that meets individuals’ needs [16,17,18,19]. Healthcare providers play a critical role in enhancing patients’ ability to adhere to treatment plans by providing recommendations, education to build knowledge, and skills training to build self-efficacy for pain and disease self-management [18,20]. Without explicit efforts to comprehensively assess and discuss pain management options, people living with frequent pain may neglect to raise issues about pain with their bleeding disorder clinicians, thinking that nothing can be done to change long-standing pain, and the care team may fail to fully understand the patient's pain management needs or constraints on their ability to follow through on recommendations [21]. Therefore, communication with patients about their treatment goals, values, and available treatment alternatives may improve treatment planning, foster self-management, and result in better patient outcomes [18]. PRO tools facilitate and expedite this communication, particularly when multiple heath care providers are involved in care provision [17].
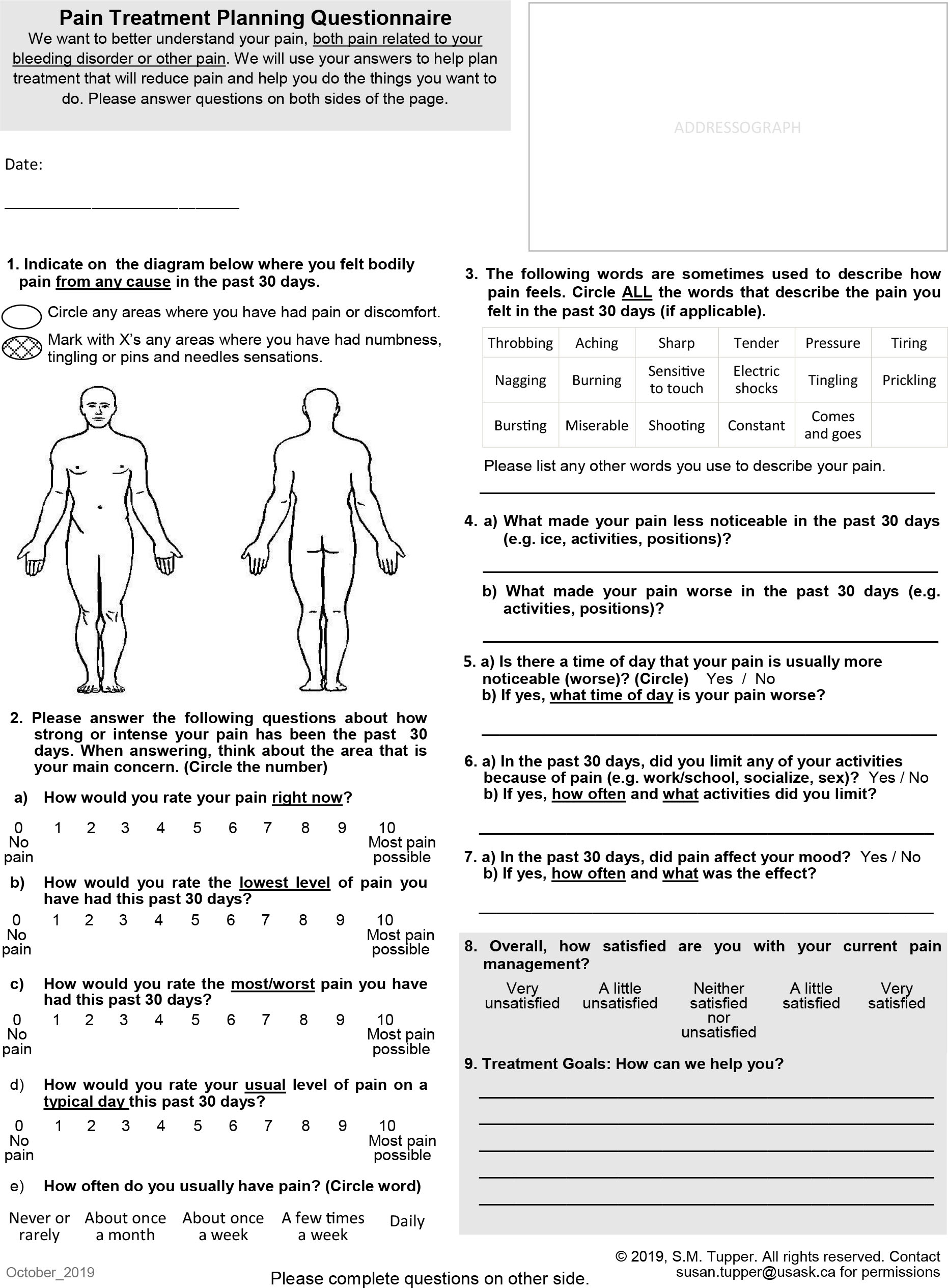
We describe the development and clinical feasibility of a condition-specific PRO tool, based on a small clinical sample, that can be used in outpatient bleeding disorder clinics to facilitate pain assessment and communication about treatment options. The Pain Treatment Planning Questionnaire (PTPQ) was co-designed with patient and multidisciplinary clinician input through an iterative process involving working group meetings, patient cognitive interviews, and examination of feasibility in a clinical setting.
METHODS
A three-step process was used for development of the PTPQ [22]:
Conceptual framework and item selection — Identify important measurement domains for the tool based on expert consensus and literature review
Item and instrument refinement — Identify and pilot test relevant domain items and measurement properties through patient cognitive interviews
Clinical feasibility evaluation — Evaluate length of time to complete, ease of understanding of the questionnaire, and participant perceptions of acceptability.
Step 1: Conceptual framework and item selection
The original idea for the PTPQ arose from a team planning meeting for a province-wide bleeding disorders program in Saskatchewan, Canada in April 2014. The meeting was attended by 27 people including four patient/family representatives, multidisciplinary bleeding disorder clinicians (four haematologist physicians, four nurses, five lab technicians, three physical therapists, and two social workers), two program managers/decision-makers, two administrative staff, and a strategy consultant on pain quality improvement. Clinicians and patient/family representatives identified pain as an important component of living with a bleeding disorder that could be more adequately addressed through improved clinical communication. A small working group was convened to develop a tool that would address this need. The working group included one patient representative, the pain strategy consultant, and seven multidisciplinary clinicians from the provincial bleeding disorders program: two haematologist physicians, three nurses, a physical therapist, and a social worker.
One team member (ST) searched for published literature on Ovid MEDLINE to identify key references for pain assessment domains and pain treatment practice guidelines for bleeding disorder populations. All search terms were expanded and included (haemophilia OR von Willebrand disease) AND pain, AND (guideline OR assess* OR evaluati*OR tool*OR instrument*OR best practice*OR recommendation*OR standard*). English language, published, peer-reviewed journal articles identified in the search were reviewed. Extracted data included pain assessment tool names, domains, and item wording or scale properties (e.g. Numeric Rating Scale, pain intensity, 0 to 10 scale, 0 is “no pain” and 10 is “most pain possible”), and a list of common medication and non-drug pain treatments (e.g. opioids, acetaminophen/paracetamol, ankle brace, cognitive behavioural therapy). Extracted data were presented to the working group for discussion and items were selected for a prototype of the PTPQ, which was created through discussion and group consensus.
Step 2: Item and instrument refinement
Research ethics approval was obtained from the University of Saskatchewan Behavioral Research Ethics Review Board (BEH 15–71). Cognitive interviews [23,24] were conducted with a purposive sample of adults with bleeding disorders between March and September 2015. The sample included participants with either haemophilia A, haemophilia B, or VWD who were known by clinician members of the research team (JK, JN, NH) to have diverse pain experiences related to their bleeding disorder. After obtaining informed consent, participants completed the PTPQ in a “think-aloud” format [23,24]. A single interviewer (ST) asked participants a set of predetermined questions with additional probing questions based on their responses during the completion of the questionnaire (see Appendix A for interview guide). This elicited a greater understanding of their interpretation of the PTPQ items and the responses provided, and suggested item revisions [25]. Interviews were audio-recorded. Two team members (ST, JK) reviewed sets of two recordings at a time, first independently, and a second time together to discuss if revisions to the PTPQ were warranted. Two team members (PD and JK) reviewed the revised PTPQ version and recordings and approved changes prior to use in the next successive pair of interviews. Interview recruitment ceased when two consecutive interviews resulted in no further substantive changes to the prototype. A clinical psychologist and pharmacist were consulted to provide feedback on the prototype since these disciplines were not represented on the questionnaire development working group. A second working group meeting was held to review and finalise the PTPQ prototype prior to use in the clinical feasibility trial. This process was used to ensure face and content validity of the questionnaire from both the patient and the clinician perspectives. After completion of the clinical feasibility evaluation, a final working group meeting was held to discuss clinical use of the PTPQ and to determine if any final changes were recommended.
Step 3: Clinical feasibility evaluation
A convenience sample of 42 patients was recruited by a non-clinician researcher (ST) from patients attending the bleeding disorder clinic between October 2015 and February 2017. Participants were adults (17 years of age and older) with a diagnosis of either haemophilia A or haemophilia B (any severity) or VWD (any type), able to read grade 8 level of English or higher, without physical or cognitive impairments that would prevent self-report. Upon obtaining informed consent, participants completed the following questionnaires:
PTPQ
Demographic questionnaire, including questions on age, sex, bleeding disorder diagnosis and severity
Chronic Pain Grade Scale (CPGS), a valid and reliable seven-item questionnaire used to categorise severity of chronic pain into five hierarchical grades according to pain intensity and pain-related disability ranging from grade 0 (no pain problem) to grade 4 (high disability that is severely limiting) [26,27]. The CPGS is recommended for use with adults with any musculoskeletal chronic pain condition [28] and has demonstrated good scale reliability (Cronbach's alpha =0.91 and average item-total correlation =0.76) [26]. Two-week test-retest reliability of the Italian version was k=0.81 [29]. Convergent validity was confirmed with highly significant associations (all p<0.001) between CPGS categories and SF-36 general health status questionnaire scores for physical, psychological, social, general health, and bodily pain.
Clinical feasibility questionnaire, including question on patient perception of difficulty/ease of understanding the PTPQ questions (five-point Likert scale), approximate time to complete the PTPQ (five categories, from “less than two minutes” to “more than ten minutes”), and five yes/no questions with an open text option for further comment on patient perceptions about feeling informed about their pain management plan, feeling that adequate information about pain was received during the clinic visit, whether any of the questions on the PTPQ were not important, whether any of the questions on the PTPQ were confusing, or whether they recommended any other changes to the questionnaire.
Associations between CPGS categories and PTPQ outcomes were tested. It was expected that participants from higher disability categories would report higher composite pain scores (average of four pain intensity questions) and would be more likely to report pain interference with activities and mood.
RESULTS
Step 1: Conceptual framework and item selection
Narrative data were extracted from 11 articles found in the literature review that described pain assessment tools or domains and recommended pain treatments. Consensus on items to include in the PTPQ prototype was achieved with a single working group meeting. These items were collated into a two-page PTPQ prototype that was used in cognitive interviews. Consultation with the psychologist and pharmacist resulted in minor revisions, including the addition of common medication brand names to improve patient recognition.
Step 2: Item and instrument refinement
Nine adults, ranging in age from 19 to 71 years (56% male), completed cognitive interviews. Participants had haemophilia A (n=4; 1 mild, 3 severe), haemophilia B (n=1, severe), and VWD (n=4). Interviews ranged in length from 30 to 90 minutes. Participants provided feedback on organisation of the questionnaire, item wording, and redundancy. For example, the initial prototype had a one-week recall period for pain severity questions, which was increased to a one-month recall to capture the pain experiences of individuals who experience pain less frequently. Further revisions to the PTPQ prototype wording were made subsequent to the working group meeting following the clinical feasibility study. These revisions included the addition of four items regarding perception of self-efficacy for self-management with different treatment categories, rated on an ordinal scale from 0 to 10, with higher numbers representing perception of better management. Since the items on perception of self-management were added after the clinical feasibility study, no data are available on these items (see Figure 1 for PTPQ development process).
Step 3: Clinical feasibility evaluation
A convenience sample of 42 adults (age range 17 to 82 years; μ=46.7 years, SD=18.3, Shapiro-Wilk Test for normal distribution p=0.066) participated in the clinical feasibility study (see Table 1 for participant characteristics). All participants meeting study inclusion criteria were invited to participate during a clinic visit by a researcher who was not a member of the multidisciplinary care team (ST). A total of 46 patients were approached; four chose to not participate due to lack of interest (91% recruitment rate). Participants had a median completion time of five to seven minutes and mean item response rate of 95.2% for the PTPQ. Questions most likely to be left blank were open text fields such as, “How often and what activities did you limit?” (50% not completed) and, “Treatment goals: how can we help you?” (35.7% not completed). The majority of participants (n=40; 95.2%) reported no difficulty understanding items. In response to the question, “Were any of the questions not important?” one participant identified the questions on emotional impact and one participant identified the prevention and physical treatments questions. In response to the question, “Were any of the questions confusing?” one patient responded, “I don’t have a lot of pain with my bleeding disorder so I don’t feel as I could fully answer the questions on pain as I don’t have any.” In response to the statement, “Please describe any changes you recommend to improve the questionnaire (e.g. change wording, add questions, remove questions),” the majority of participants either left the question blank or responded with positive feedback, such as “It is clear and easy to fill out,” or “Great info and questions.” One participant suggested increasing the amount of space to allow more room to explain responses to questions.
Table 1
Clinical feasibility study participant characteristics (n=42)
Pain characteristics reported on the PTPQ
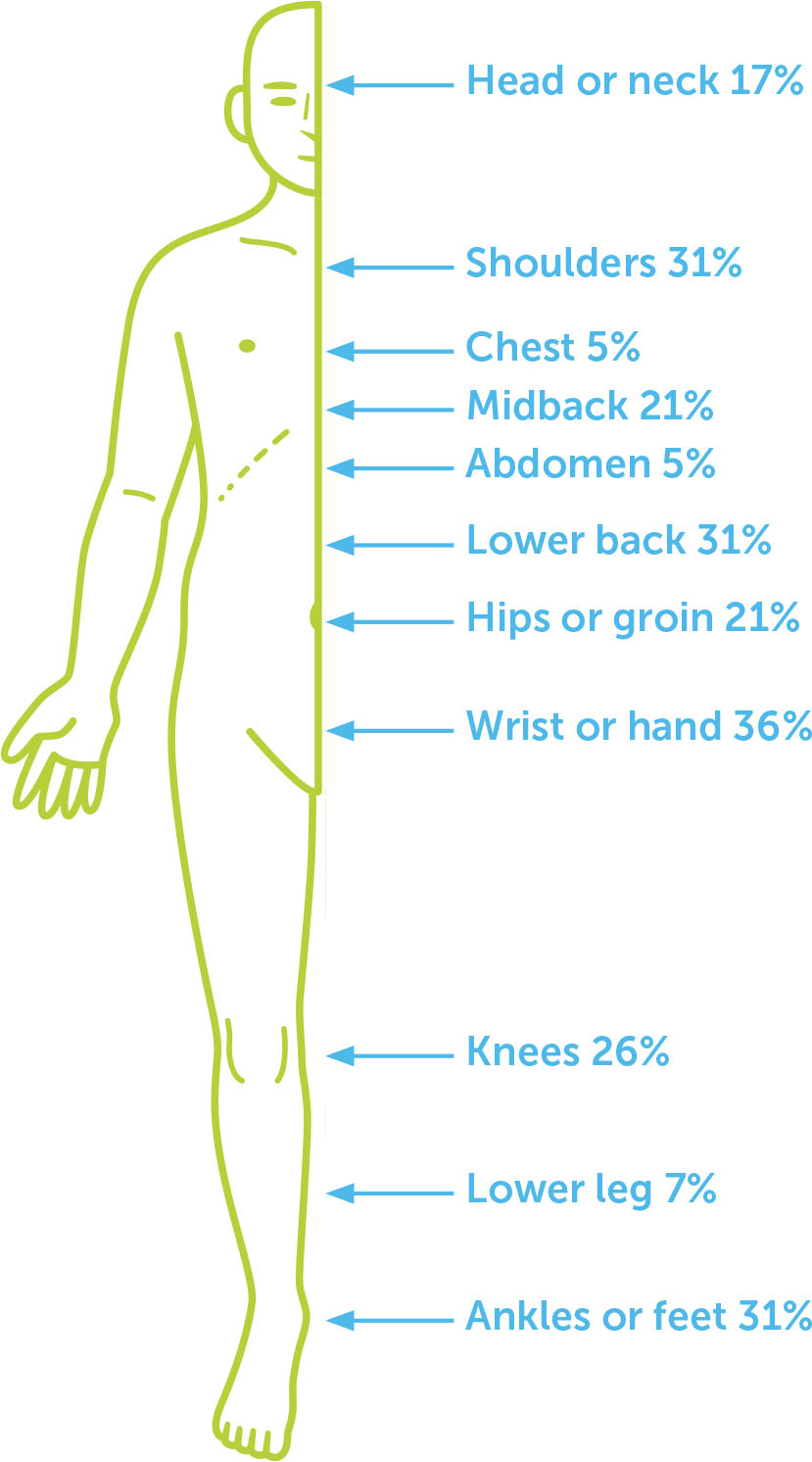
The majority (73.8 %) of participants reported pain at the time of the study (non-zero score for the “current pain” question), with a mean score of 2.3 (range 0 = “No pain” to 10 = “Most pain possible”) to the question, “How would you rate your pain right now?” The majority of participants (57.1%) reported persistent pain in the past month, defined as a non-zero score to the question, “How would you rate the lowest level lowest of pain you have had this past 30 days?” (see Table 2 for pain characteristics identified on the PTPQ). The body locations of pain most frequently selected on the diagram in response to the question “Indicate on the diagram below where you felt bodily pain from any cause in the past 30 days,” were the forearm, wrist, or hands (36% of total sample), and the lower back, shoulders, ankles or feet (31% of total sample for each area; see Figure 2 for locations selected). The majority of participants (71.4%) identified two or more locations of pain on the body diagram. The most common descriptive words selected were “aching” (67% of total sample) and “sharp” (38%). Almost half (47.6%) of participants selected at least one word typically used to describe neuropathic pain and paraesthesia symptoms, e.g. burning, electric shocks, tingling, prickling, bursting, shooting. Almost half of the participants did not report pain interference with activities or mood (47.6%), 33.3% reported interference with both, and 14.3% reported interference with either activities or mood (4.8% missing).
Table 2
Pain characteristics identified on PTPQ (sample n=42 unless otherwise specified)
The most common open-text response to the question “What made your pain less noticeable in the past 30 days?” were activities (n=19; 45.2% of all participants) such as “keeping active” or “exercises”. In response to the question, “What made your pain worse in the past 30 days?” participants provided equal frequencies of responses (n=17; 40.5%) for activity or work (e.g. “too much activity”) and prolonged positioning (e.g. “prolonged sitting”). Over half of all participants reported a typical daily pattern to pain intensity, with the majority reporting worst pain in the evening (n=9; 21.4%) or morning (n=7; 16.7%).
Due to low numbers in CPGS categories, a dichotomous variable was created for no pain/low disability (CPGS categories 0–2) and high disability (CPGS categories 3 and 4). Composite pain scores in both groups were normally distributed and had equality of variance. Mean composite pain scores were significantly different between dichotomous CPGS groups on Independent Samples T-test (μ pain=2.6 [1.6] no pain/low disability; μ pain=4.4 [2.2] high disability; p=0.013, CI=0.41, 3.26). Trends towards group differences in proportions of participants reporting mood and activity interference between no pain/low disability and high disability groups were identified (Table 3). Although the statistical tests are reported, these results can only be interpreted as trends and should be interpreted with caution due to the unacceptably low numbers in individual cells.
Table 3
Participants reporting activity limitations or mood interference within CPGS clusters
| CPGS CATEGORY | SIG. * | |||
|---|---|---|---|---|
| NO PAIN/LOW DISABILITY (CPGS 0–2) | HIGH DISABILITY (CPGS 3–4) | |||
| Activity limitations from pain | No | 23 | 2 | p=0.081 |
| Yes | 10 | 5 | ||
| Mood interference from pain | No | 21 | 1 | p=0.041 |
| Yes | 14 | 6 | ||
DISCUSSION
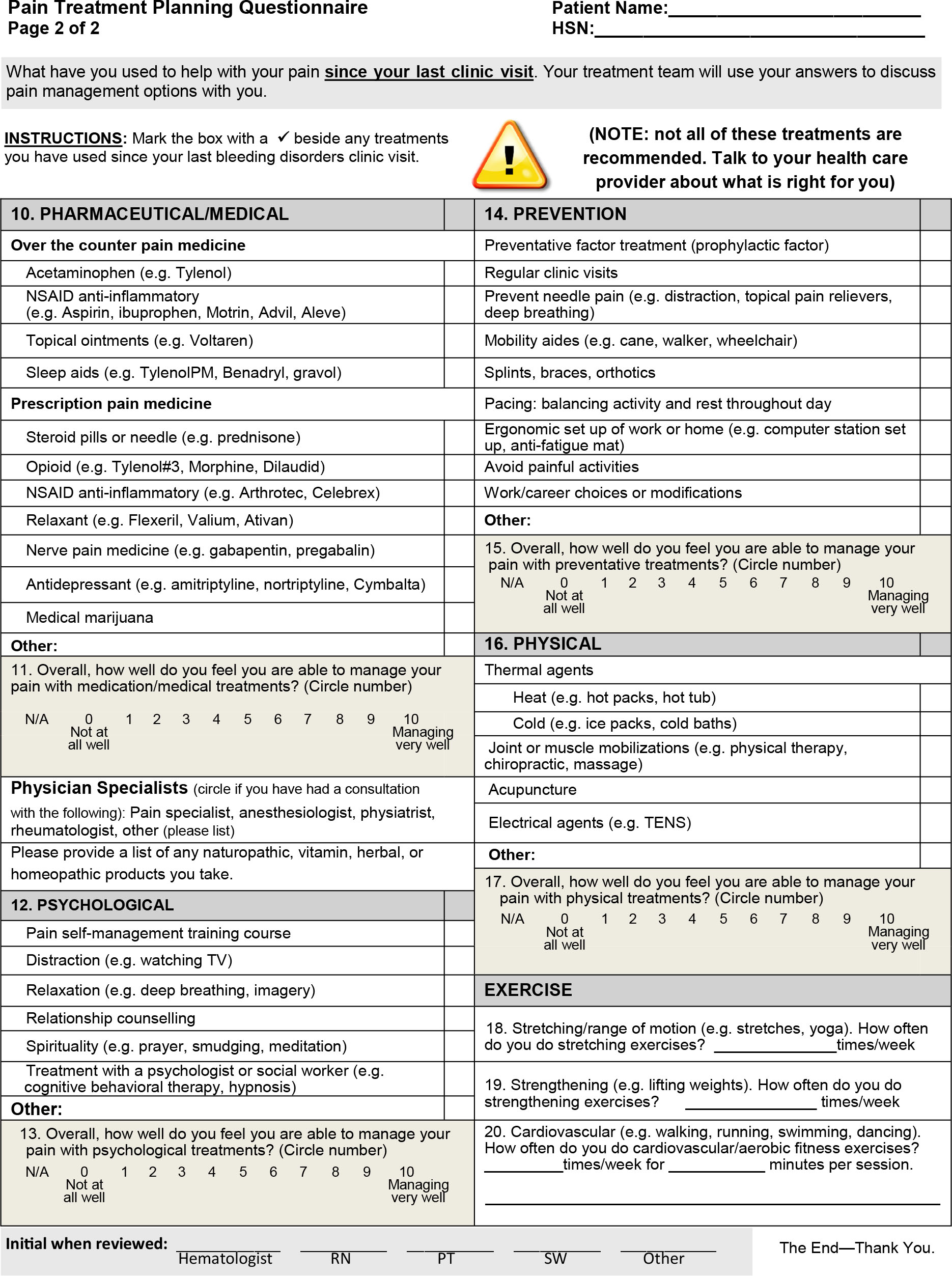
The PTPQ is a 28-item condition-specific PRO instrument developed for use in the outpatient setting to guide communication about pain and pain treatment between patients with bleeding disorders and multidisciplinary healthcare providers (see Appendix B). It is a multidimensional tool that measures a number of constructs, including body locations of pain, pain severity, pain frequency, descriptive qualities, pain interference, palliating and provoking factors, timing, satisfaction with treatment, a checklist of treatments, exercise participation, and self-efficacy to manage pain with different categories of treatment. Similar to any focused pain history, these different constructs can be used to guide further investigations into the underlying mechanisms of pain (i.e. nociceptive vs. neuropathic), to better understand the impact of pain on the individual, and guide pain treatment planning. Given the high prevalence and impact of pain in this population described in previous studies [2,3,4,9,30,31] and the current sample, as well as patient reports of dissatisfaction with pain management described in previous research [4], discussion about pain at regular clinic visits should be considered a critical component of person-centred care. Use of a tool, such as the PTPQ, to facilitate discussion about pain may improve clinicians’ understanding of patient pain experiences and patient understanding of appropriate treatment and self-management options [18,19].
A general population survey conducted in Germany by Hauser et al. (2015) found that 71.5% of those surveyed did not have chronic pain, compared to only 2.4% of the current clinical sample who reported no chronic pain [32]. The survey reported that 19.1% had non-disabling chronic pain (CPGS scores 1 or 2), and 7.3% had disabling chronic pain (CPGS scores 3 or 4), compared to the current study in which 81.0% had non-disabling chronic pain, and 16.6% had disabling chronic pain. Higher prevalence of chronic pain that interferes with physical function among those with haemophilia has been previously reported. In a survey of participants with haemophilia A or B in which 57% reported daily pain, Elander et al. (2009) found that the impact of pain intensity on physical quality of life was mediated by pain beliefs and behaviours (i.e. pain willingness subscale of the Chronic Pain Acceptance Questionnaire), demonstrating that presence of chronic pain alone is not a predictor of impact on physical function [1]. Forsyth et al. (2015) found that 89% of participants with haemophilia in an international sample reported that pain interfered to some extent with their activities in the previous month, with 26% reporting higher levels of interference and 21% reporting persistent pain [3].
Previous research has demonstrated that people with chronic pain prioritise care that accounts for their personal situation and capacity to follow recommendations and is oriented towards achievement of their personal goals [33]. Street et al. propose that communication between patients and providers indirectly affects health outcomes such as pain intensity and interference by influencing patients’ self-efficacy to manage their condition [16]. In a study primarily involving male veterans with chronic pain, Ruben et al. (2018) found that more positive perceptions of patient-provider communication were associated with lower pain intensity and pain interference scores and higher levels of self-efficacy to manage their health [20]. Although directionality of these relationships cannot be determined from that study, positive communication, with instruction on self-management skills, reassurance, and encouragement, could be expected to influence self-efficacy to self-manage and thereby have a positive impact on pain management. Simply stated, communication about pain management can have a therapeutic effect.
The PTPQ is intended to be used as a guide to facilitate discussions about pain management options as a means of promoting self-efficacy in self-management skills. As such, it was structured to collect a focused pain history including multidimensional elements recommended in pain assessment clinical practice guidelines [34,35,36]. Each of the treatment team members may address different sections of the questionnaire with the patient, thereby streamlining the interactions about pain and reducing the need for patients to repeat their description of the pain experience and treatments used. The PTPQ includes a checklist of common pain treatments which clinicians can use to determine whether the patient is using a broad range of recommended treatments, including both pharmacologic and nonpharmacologic strategies. If treatment benefits are achieved as a result of the use of the PTPQ, this could be attributable to the discussion of treatment options, self-assessment of capacity to use the treatment, and problem solving through challenges identified. The treatment checklist could be used to guide members of the multidisciplinary care team to provide education and supportive interventions to promote self-management. The PTPQ could also be used to open discussions about the patient's capacity to follow treatment recommendations and determine if the patient requires additional care, resources, education or training to manage pain. The need for referrals to community-based providers, such as mental healthcare providers or physician specialists (e.g. pain specialists or multidisciplinary pain clinics), and support for resource access could also be identified and referrals expedited.
A qualitative approach was used to ensure that the PTPQ was developed in a manner that would maximise content validity. This approach has been recognised in the literature as an acceptable and patient-oriented process for tool development [37]. Brod et al. (2009) define content validity as “the measurement property that assesses whether items are comprehensive and adequately reflect the patient perspective for the population of interest. In addition, content validity provides evidence that formatting, instructions and response options are relevant, and the measure is understandable and acceptable to patients.” Given that components of a focused pain history are well established and have been described in clinical practice guidelines, the research team did not feel that a rigorous grounded theory approach was necessary for item development. Cognitive interviews were conducted with patient participants until no further substantive changes were identified. The PTPQ was then approved by the multidisciplinary working group and consultant psychologist and pharmacist. Over 95% of the clinical sample surveyed had no suggested revisions and reported that the PTPQ contained all relevant items for clinical communication about pain. It should be noted, however, that content validity cannot truly be said to occur until the measure shows responsiveness to change with a successful intervention. Further research will be required to examine this.
The PTPQ demonstrates reasonable clinical feasibility with a relatively low median completion time and high item response rate. Two participants who did not report pain found the questions on the PTPQ to be confusing. When providing the questionnaire for completion in the clinic setting, clinicians could prevent this confusion by informing patients on how to respond to the questions if they have no pain (e.g. leave the body diagram blank, circle 0 on the pain intensity questions). The working group decided to not implement changes recommended to the PTPQ by participants regarding an increase in the amount of response space to allow patients to explain their responses in more depth. Since the open text questions were left blank by half of participants, and since the PTPQ is intended to facilitate clinical discussion, the group decided that these recommendations would increase the number of pages or time required to complete the questionnaire and likely not enhance the value of the questionnaire for most patients and clinicians.
Strengths and limitations of the research
The PTPQ is unique from other general multidimensional pain assessment tools, such as the McGill Pain Questionnaire [38] and Brief Pain Inventory [39], in that it is condition-specific and contains a multimodal treatment checklist. Strengths of the PTPQ include development through a co-design approach using broad engagement of both patients and clinicians in item selection and refinement through working group meetings, cognitive interviews, and clinical feasibility testing which followed an established tool development framework. A limitation of this research is that tool development and feasibility testing were conducted in one clinic with a small number of patient participants. It should be noted, however, that this clinic serves the entire bleeding disorder population of the Saskatchewan province, and the clinicians involved are leaders and active members of discipline-specific working groups of the Canadian Hemophilia Society (e.g. Canadian Physiotherapists in Hemophilia Care). The PTPQ is in the early stages of development and should be subject to further research to determine if it is valid and reliable and achieves its intended objectives of supporting clinical communication about pain.
Future directions
Further research is needed to determine if the PTPQ is clinically acceptable outside of the clinic and province in which it was developed. This research should also examine clinician perspectives on the clinical utility of the PTPQ, and whether use of the PTPQ impacts clinical decision-making. An Implementation Science approach [40] to exploring the contextualisation of the PTPQ and the organisational characteristics that predict how the PTPQ is applied in different clinics will inform recommendations for application. Future research will need to determine if the PTPQ impacts clinical communication between patients and healthcare providers and between multidisciplinary healthcare team members, and whether communication is a mediating factor that influences pain outcomes. In turn, this would inform future research to support aspects of communication that most effectively optimise pain outcomes.
The composite pain score on the PTPQ was associated in the expected direction with disability status measured with the CPGS. Although the PTPQ is not intended as a diagnostic tool for measurement of a specific construct, further psychometric testing of the PTPQ is warranted to determine its reliability and validity. Clinical teams should be aware of potential limitations of implementing the PTPQ given it is in early stages of psychometric testing.
CONCLUSIONS
Recent calls to action have recommended development of standardised approaches to management of pain in patients with haemophilia [6,8]. This would necessarily include improving the capacity of clinicians in bleeding disorder treatment clinics to assess, educate, and communicate with patients about pain. The PTPQ is a clinically feasible PRO measure that shows promise for assessing the multidimensional nature of pain in adults with bleeding disorders in outpatient settings and facilitating communication between patients and clinicians about condition-specific pain treatment options. Further research is needed to evaluate the clinical utility and reliability of the PTPQ as well as to determine if communication about pain with the PTPQ affects pain outcomes in patients.